
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
Review
- Basic Research
- Heterogeneity of Islet Cells during Embryogenesis and Differentiation
- Shugo Sasaki, Takeshi Miyatsuka
- Diabetes Metab J. 2023;47(2):173-184. Published online January 12, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0324
- 3,760 View
- 248 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
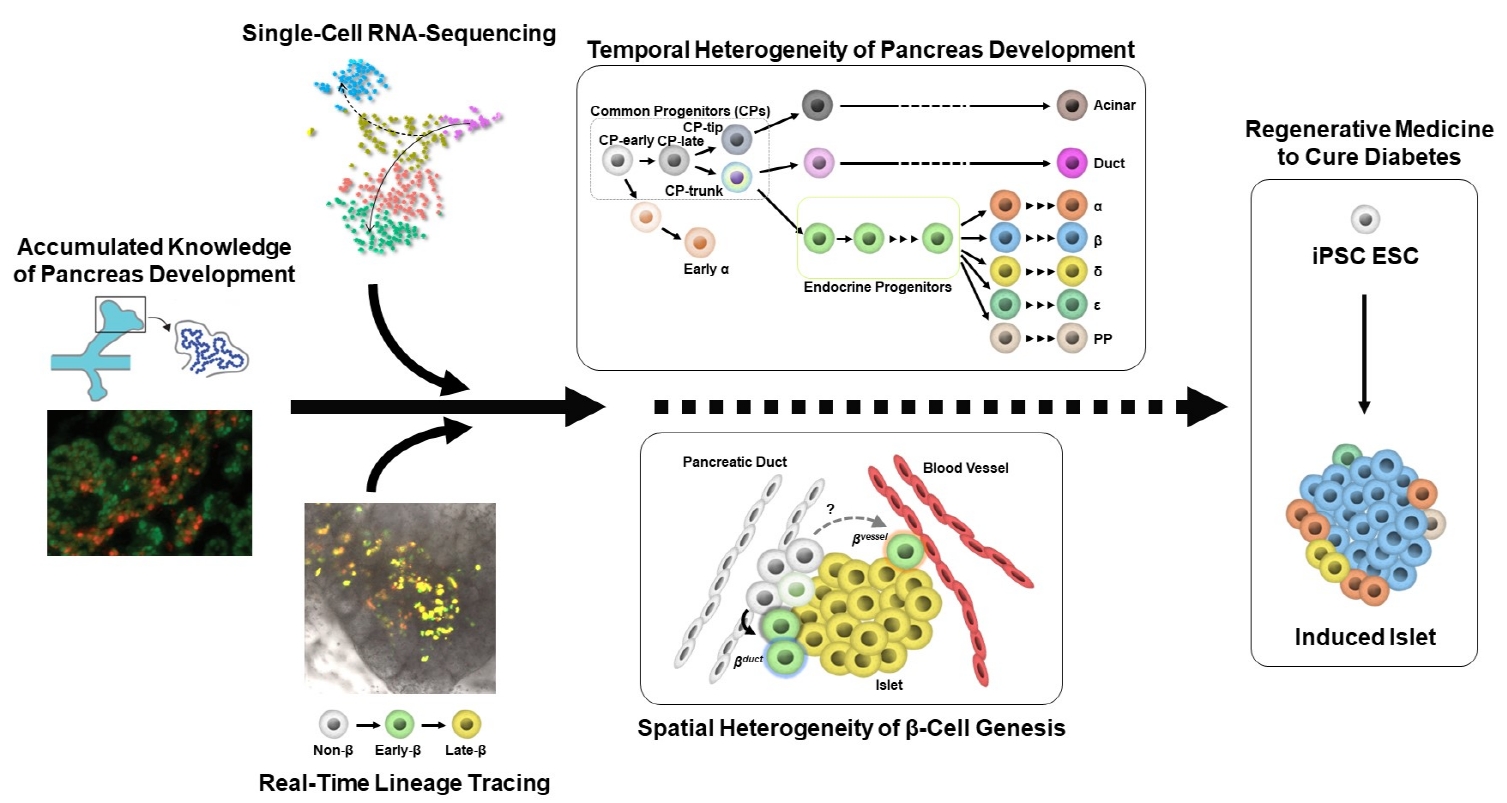
ePub - Diabetes is caused by insufficient insulin secretion due to β-cell dysfunction and/or β-cell loss. Therefore, the restoration of functional β-cells by the induction of β-cell differentiation from embryonic stem (ES) and induced-pluripotent stem (iPS) cells, or from somatic non-β-cells, may be a promising curative therapy. To establish an efficient and feasible method for generating functional insulin-producing cells, comprehensive knowledge of pancreas development and β-cell differentiation, including the mechanisms driving cell fate decisions and endocrine cell maturation is crucial. Recent advances in single-cell RNA sequencing (scRNA-seq) technologies have opened a new era in pancreas development and diabetes research, leading to clarification of the detailed transcriptomes of individual insulin-producing cells. Such extensive high-resolution data enables the inference of developmental trajectories during cell transitions and gene regulatory networks. Additionally, advancements in stem cell research have not only enabled their immediate clinical application, but also has made it possible to observe the genetic dynamics of human cell development and maturation in a dish. In this review, we provide an overview of the heterogeneity of islet cells during embryogenesis and differentiation as demonstrated by scRNA-seq studies on the developing and adult pancreata, with implications for the future application of regenerative medicine for diabetes.
-
Citations
Citations to this article as recorded by- Newly discovered knowledge pertaining to glucagon and its clinical applications
Dan Kawamori, Shugo Sasaki
Journal of Diabetes Investigation.2023; 14(7): 829. CrossRef
- Newly discovered knowledge pertaining to glucagon and its clinical applications
Original Article
- Transdifferentiation of Enteroendocrine K-cells into Insulin-expressing Cells.
- Esder Lee, Jun Mo Yu, Min Kyung Lee, Gyeong Ryul Ryu, Seung Hyun Ko, Yu Bae Ahn, Sung Dae Moon, Ki Ho Song
- Korean Diabetes J. 2009;33(6):475-484. Published online December 1, 2009
- DOI: https://doi.org/10.4093/kdj.2009.33.6.475
- 2,222 View
- 19 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Despite a recent breakthough in human islet transplantation for treating type 1 diabetes mellitus, the limited availability of donor pancreases remains a major obstacle. Endocrine cells within the gut epithelium (enteroendocrine cells) and pancreatic beta cells share similar pathways of differentiation during embryonic development. In particular, K-cells that secrete glucose-dependent insulinotropic polypeptide (GIP) have been shown to express many of the key proteins found in beta cells. Therefore, we hypothesize that K-cells can be transdifferentiated into beta cells because both cells have remarkable similarities in their embryonic development and cellular phenotypes. METHODS: K-cells were purified from heterogeneous STC-1 cells originating from an endocrine tumor of a mouse intestine. In addition, a K-cell subclone expressing stable Nkx6.1, called "Kn4-cells," was successfully obtained. In vitro differentiation of K-cells or Kn4-cells into beta cells was completed after exendin-4 treatment and serum deprivation. The expressions of insulin mRNA and protein were examined by RT-PCR and immunocytochemistry. The interacellular insulin content was also measured. RESULTS: K-cells were found to express glucokinase and GIP as assessed by RT-PCR and Western blot analysis. RT-PCR showed that K-cells also expressed Pdx-1, NeuroD1/Beta2, and MafA, but not Nkx6.1. After exendin-4 treatment and serum deprivation, insulin mRNA and insulin or C-peptide were clearly detected in Kn4-cells. The intracellular insulin content was also increased significantly in these cells. CONCLUSION: K-cells are an attractive potential source of insulin-producing cells for treatment of type 1 diabetes mellitus. However, more experiments are necessary to optimize a strategy for converting K-cells into beta cells. -
Citations
Citations to this article as recorded by- Reprogramming of enteroendocrine K cells to pancreatic β-cells through the combined expression of Nkx6.1 and Neurogenin3, and reaggregation in suspension culture
Esder Lee, Gyeong Ryul Ryu, Sung-Dae Moon, Seung-Hyun Ko, Yu-Bae Ahn, Ki-Ho Song
Biochemical and Biophysical Research Communications.2014; 443(3): 1021. CrossRef
- Reprogramming of enteroendocrine K cells to pancreatic β-cells through the combined expression of Nkx6.1 and Neurogenin3, and reaggregation in suspension culture
Review
- Stimulation of Glucagon Like Peptide-1 Secretion in Enteroendocrine L cells.
- Byung Joon Kim
- Korean Diabetes J. 2009;33(6):458-463. Published online December 1, 2009
- DOI: https://doi.org/10.4093/kdj.2009.33.6.458
- 1,896 View
- 20 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - GLP-1 (glucagon like peptide-1) is new anti-diabetic drug with a number of beneficial effects. It stimulates glucose dependant insulin secretion and restoration of beta cell mass through enhancement of islet mass. However, it is easily inactivated after being secreted from enteroendocrine L cells. Recent trial to increased GLP-1 is to directly stimulate L cells through its receptor located in the surface of L cell. Taste receptor in the apical surface of L cell is activated by various tastants contained in the food. Tongue perceives taste sense through the heterotrimeric G-protein (alpha-gustducin) and its downstream signaling cascades. Same taste receptors are also expressed in enteroendocrine cells. In duodenal L cell, alpha-gustducin was detected by immunofluorescence stainig at the luminal projections of enteroendocrine cells. And several other taste signaling elements were also found in L cells. Ingestion of sweet or bitter compounds revealed stimulation of GLP-1 secretion and the regulation of plasma insulin and glucose. In this review, I will briefly introduce the possibilities to stimulate GLP-1 secretion though the membrane receptor in enteroendocrine cell. And it will be the good candidate to develop the treatment modality for obesity, diabetes and abnormal gut motility.
-
Citations
Citations to this article as recorded by- Repression of sterol regulatory element-binding protein 1-c is involved in the protective effects of exendin-4 in pancreatic β-cell line
Seok-Woo Hong, Jinmi Lee, Se Eun Park, Eun-Jung Rhee, Cheol-Young Park, Ki-Won Oh, Sung-Woo Park, Won-Young Lee
Molecular and Cellular Endocrinology.2012; 362(1-2): 242. CrossRef - Exendin-4 Protects Oxidative Stress-Induced β-Cell Apoptosis through Reduced JNK and GSK3β Activity
Ju-Young Kim, Dong-Mee Lim, Chan Il Moon, Kyung-Jin Jo, Seong-Kyu Lee, Haing-Woon Baik, Ki-Ho Lee, Kang-Woo Lee, Keun-Young Park, Byung-Joon Kim
Journal of Korean Medical Science.2010; 25(11): 1626. CrossRef
- Repression of sterol regulatory element-binding protein 1-c is involved in the protective effects of exendin-4 in pancreatic β-cell line

 KDA
KDA
 First
First Prev
Prev





