
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Guideline/Fact Sheet
- Dyslipidemia Fact Sheet in South Korea, 2022
- Eun-Sun Jin, Jee-Seon Shim, Sung Eun Kim, Jae Hyun Bae, Shinae Kang, Jong Chul Won, Min-Jeong Shin, Heung Yong Jin, Jenny Moon, Hokyou Lee, Hyeon Chang Kim, In-Kyung Jeong, on Behalf of the Committee of Public Relation of the Korean Society of Lipid and Atherosclerosis
- Diabetes Metab J. 2023;47(5):632-642. Published online August 2, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0135
- 3,098 View
- 319 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
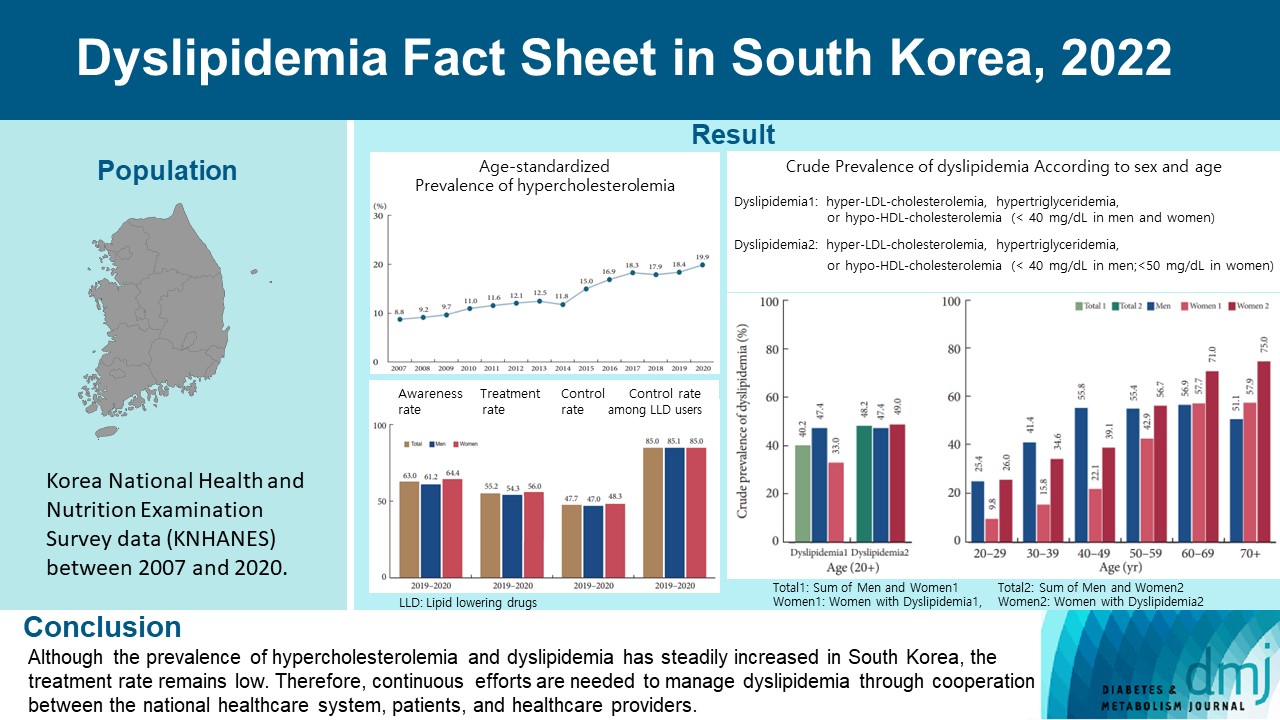
This study aimed to investigate the prevalence and status of dyslipidemia management among South Korean adults, as performed by the Korean Society of Lipid and Atherosclerosis under the name Dyslipidemia Fact Sheet 2022.
Methods
We analyzed the lipid profiles, age-standardized and crude prevalence, management status of hypercholesterolemia and dyslipidemia, and health behaviors among Korean adults aged ≥20 years, using the Korea National Health and Nutrition Examination Survey data between 2007 and 2020.
Results
In South Korea, the crude prevalence of hypercholesterolemia (total cholesterol ≥240 mg/dL or use of a lipid-lowering drug) in 2020 was 24%, and the age-standardized prevalence of hypercholesterolemia more than doubled from 2007 to 2020. The crude treatment rate was 55.2%, and the control rate was 47.7%. The crude prevalence of dyslipidemia—more than one out of three conditions (low-density lipoprotein cholesterol ≥160 or the use of a lipid-lowering drug, triglycerides ≥200, or high-density lipoprotein cholesterol [HDL-C] [men and women] <40 mg/dL)—was 40.2% between 2016 and 2020. However, it increased to 48.2% when the definition of hypo-HDL-cholesterolemia in women changed from <40 to <50 mg/dL.
Conclusion
Although the prevalence of hypercholesterolemia and dyslipidemia has steadily increased in South Korea, the treatment rate remains low. Therefore, continuous efforts are needed to manage dyslipidemia through cooperation between the national healthcare system, patients, and healthcare providers. -
Citations
Citations to this article as recorded by- Oxidative Balance Score and New-Onset Type 2 Diabetes Mellitus in Korean Adults without Non-Alcoholic Fatty Liver Disease: Korean Genome and Epidemiology Study-Health Examinees (KoGES-HEXA) Cohort
Mid-Eum Moon, Dong Hyuk Jung, Seok-Jae Heo, Byoungjin Park, Yong Jae Lee
Antioxidants.2024; 13(1): 107. CrossRef - Comparison of metabolic and neurological comorbidities in Asian patients with psoriasis and atopic dermatitis
Hee Joo Yang, Mi Young Lee, Jeong Hyeon Lee, Chang Jin Jung, Woo Jin Lee, Chong Hyun Won, Mi Woo Lee, Joon Min Jung, Sung Eun Chang
Scientific Reports.2024;[Epub] CrossRef - Effect of Adding Apolipoprotein B Testing on the Prevalence of Dyslipidemia and Risk of Cardiovascular Disease in the Korean Adult Population
Rihwa Choi, Sang Gon Lee, Eun Hee Lee
Metabolites.2024; 14(3): 169. CrossRef - Exploring Utilization and Establishing Reference Intervals for the Apolipoprotein B Test in the Korean Population
Rihwa Choi, Sang Gon Lee, Eun Hee Lee
Diagnostics.2023; 13(20): 3194. CrossRef
- Oxidative Balance Score and New-Onset Type 2 Diabetes Mellitus in Korean Adults without Non-Alcoholic Fatty Liver Disease: Korean Genome and Epidemiology Study-Health Examinees (KoGES-HEXA) Cohort
- Complications
- Dyslipidemia in Patients with Chronic Kidney Disease: An Updated Overview
- Sang Heon Suh, Soo Wan Kim
- Diabetes Metab J. 2023;47(5):612-629. Published online July 24, 2023
- DOI: https://doi.org/10.4093/dmj.2023.0067

- 3,108 View
- 410 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Dyslipidemia is a potentially modifiable cardiovascular risk factor. Whereas the recommendations for the treatment target of dyslipidemia in the general population are being more and more rigorous, the 2013 Kidney Disease: Improving Global Outcomes clinical practice guideline for lipid management in chronic kidney disease (CKD) presented a relatively conservative approach with respect to the indication of lipid lowering therapy and therapeutic monitoring among the patients with CKD. This may be largely attributed to the lack of high-quality evidence derived from CKD population, among whom the overall feature of dyslipidemia is considerably distinctive to that of general population. In this review article, we cover the characteristic features of dyslipidemia and impact of dyslipidemia on cardiovascular outcomes in patients with CKD. We also review the current evidence on lipid lowering therapy to modify the risk of cardiovascular events in this population. We finally discuss the association between dyslipidemia and CKD progression and the potential strategy to delay the progression of CKD in relation to lipid lowering therapy.
-
Citations
Citations to this article as recorded by- Statin Therapy and Lipid Indices in Chronic Kidney Disease: A Systematic
Review and Meta-analysis of Randomized Control Trials
Jafar Karami, Bahman Razi, Danyal Imani, Saeed Aslani, Mahdi Pakjoo, Mahdieh Fasihi, Keyhan Mohammadi, Amirhossein Sahebkar
Current Pharmaceutical Design.2024; 30(5): 362. CrossRef
- Statin Therapy and Lipid Indices in Chronic Kidney Disease: A Systematic
Review and Meta-analysis of Randomized Control Trials
- Cardiovascular Risk/Epidemiology
- Cardiovascular Outcomes according to Comorbidities and Low-Density Lipoprotein Cholesterol in Korean People with Type 2 Diabetes Mellitus
- Min Kyong Moon, Junghyun Noh, Eun-Jung Rhee, Sang Hyun Park, Hyeon Chang Kim, Byung Jin Kim, Hae Jin Kim, Seonghoon Choi, Jin Oh Na, Young Youl Hyun, Bum Joon Kim, Kyung-Do Han, In-Kyung Jeong, on Behalf of the Committee of Practice Guideline of Korean Lipid and Atheroscelerosis
- Diabetes Metab J. 2023;47(1):45-58. Published online January 26, 2023
- DOI: https://doi.org/10.4093/dmj.2021.0344

- 2,970 View
- 262 Download
- 4 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
There are no clear data to support the cardiovascular (CV) risk categories and low-density lipoprotein cholesterol (LDL-C) treatment goals in Korean people with type 2 diabetes mellitus (T2DM). We evaluated the incidence of cardiovascular disease (CVD) according to comorbidities and suggested LDL-C treatment goals in Korean people with T2DM in nationwide cohort data.
Methods
Using the Korean National Health Insurance Service database, 248,002 people aged 30 to 90 years with T2DM who underwent routine health check-ups during 2009 were included. Subjects with previous CVD were excluded from the study. The primary outcome was incident CVD, defined as a composite of myocardial infarction and ischemic stroke during the follow-up period from 2009 to 2018.
Results
The mean age of the study participants was 59.6±10.9 years, and median follow-up period was 9.3 years. CVD incidence increased in the order of DM duration of 5 years or more (12.04/1,000 person-years), hypertension (HT) (12.27/1,000 personyears), three or more CV risk factors (14.10/1,000 person-years), and chronic kidney disease (18.28/1,000 person-years). The risk of incident CVD increased linearly from an LDL-C level of ≥70 mg/dL in most patients with T2DM. In T2DM patients without HT or with a DM duration of less than 5 years, the CVD incidence increased from LDL-C level of ≥100 mg/dL.
Conclusion
For primary prevention of CVD in Korean adults with T2DM, it can be helpful to lower LDL-C targets when there are chronic kidney disease, HT, a long duration of diabetes mellitus, or three or more CV risk factors. -
Citations
Citations to this article as recorded by- Lipid Management in Korean People with Type 2 Diabetes Mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement
Ye Seul Yang, Hack-Lyoung Kim, Sang-Hyun Kim, Min Kyong Moon
Diabetes & Metabolism Journal.2023; 47(1): 1. CrossRef - Optimal Low-Density Lipoprotein Cholesterol Level for Primary Prevention in Koreans with Type 2 Diabetes Mellitus
Ji Yoon Kim, Nam Hoon Kim
Diabetes & Metabolism Journal.2023; 47(1): 42. CrossRef - Lipid Management in Korean People With Type 2 Diabetes Mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement
Ye Seul Yang, Hack-Lyoung Kim, Sang-Hyun Kim, Min Kyong Moon
Journal of Lipid and Atherosclerosis.2023; 12(1): 12. CrossRef - 2023 Clinical Practice Guidelines for Diabetes: Management of Cardiovascular Risk Factors
Ye Seul Yang
The Journal of Korean Diabetes.2023; 24(3): 135. CrossRef - 2023 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association
Jong Han Choi, Kyung Ae Lee, Joon Ho Moon, Suk Chon, Dae Jung Kim, Hyun Jin Kim, Nan Hee Kim, Ji A Seo, Mee Kyoung Kim, Jeong Hyun Lim, YoonJu Song, Ye Seul Yang, Jae Hyeon Kim, You-Bin Lee, Junghyun Noh, Kyu Yeon Hur, Jong Suk Park, Sang Youl Rhee, Hae J
Diabetes & Metabolism Journal.2023; 47(5): 575. CrossRef - Management of Dyslipidemia in Patients with Diabetes Mellitus
Kyung Ae Lee
The Journal of Korean Diabetes.2023; 24(3): 111. CrossRef - Significant Gap Between Guidelines and Practice in the Management of LDL Cholesterol: Insight From the Survey of the Korean Society of Myocardial Infarction
Sang Yeub Lee, Kyung Hoon Cho, Jang Hoon Lee, Young Joon Hong, Jin yong Hwang, Myung Ho Jeong, Weon Kim
Journal of Korean Medical Science.2023;[Epub] CrossRef
- Lipid Management in Korean People with Type 2 Diabetes Mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement
- Guideline/Fact Sheet
- Lipid Management in Korean People with Type 2 Diabetes Mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement
- Ye Seul Yang, Hack-Lyoung Kim, Sang-Hyun Kim, Min Kyong Moon, on Behalf of Committee of Clinical Practice Guideline, Korean Diabetes Association and Clinical Practice Guideline Committee, Korean Society of Lipid and Atherosclerosis
- Diabetes Metab J. 2023;47(1):1-9. Published online January 20, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0448
- 3,577 View
- 378 Download
- 3 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
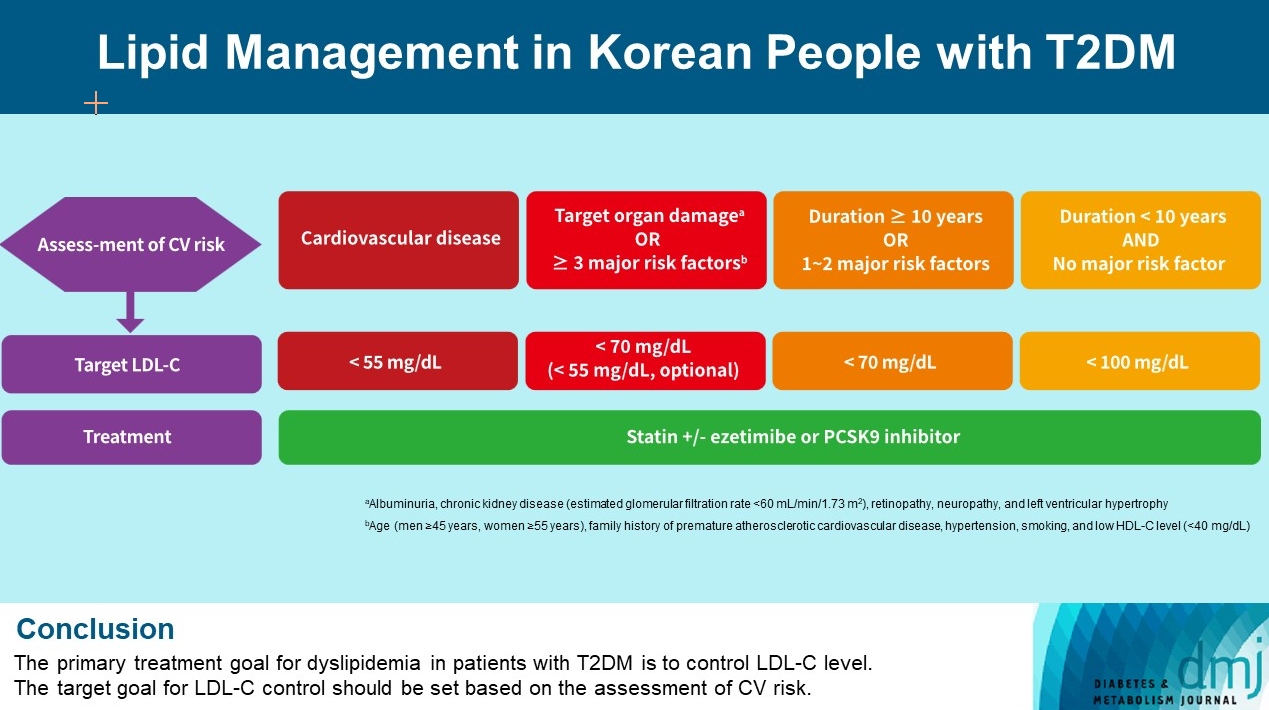
ePub - Dyslipidemia in patients with diabetes is an important treatment target as a modifiable risk factor for cardiovascular disease (CVD). Although the primary treatment goal for dyslipidemia is to control low-density lipoprotein cholesterol (LDL-C), achieving this goal remains suboptimal according to recent studies. It is important to set the target goal for LDL-C control based on an accurate risk assessment for CVD. Here, we summarize the latest evidence on lipid management in patients with diabetes and present a consensus of the Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis on the treatment goals of LDL-C according to the duration of diabetes, presence of CVD, target organ damage, or major cardiovascular risk factors. In patients with type 2 diabetes mellitus (T2DM) and CVD, an LDL-C goal of <55 mg/dL and a reduction in LDL-C level by 50% or more from the baseline is recommended. For the primary prevention of CVD in patients with T2DM with a duration of diabetes ≥10 years, major cardiovascular risk factors, or target organ damage, an LDL-C goal of <70 mg/dL is recommended. In patients with T2DM with a duration of diabetes <10 years and no major cardiovascular risk factors, an LDL-C goal of <100 mg/dL is recommended.
-
Citations
Citations to this article as recorded by- Associations of omega-3 fatty acids vs. fenofibrate with adverse cardiovascular outcomes in people with metabolic syndrome: propensity matched cohort study
Nam Hoon Kim, Ji Yoon Kim, Jimi Choi, Sin Gon Kim
European Heart Journal - Cardiovascular Pharmacotherapy.2024; 10(2): 118. CrossRef - Clinical Characteristics of Patients With Statin Discontinuation in Korea: A Nationwide Population-Based Study
Kyung-Soo Kim, Sangmo Hong, Kyungdo Han, Cheol-Young Park
Journal of Lipid and Atherosclerosis.2024; 13(1): 41. CrossRef - Role of Fenofibrate Use in Dyslipidemia and Related Comorbidities in the Asian Population: A Narrative Review
Chaicharn Deerochanawong, Sin Gon Kim, Yu-Cheng Chang
Diabetes & Metabolism Journal.2024; 48(2): 184. CrossRef - Dyslipidemia Fact Sheet in South Korea, 2022
Eun-Sun Jin, Jee-Seon Shim, Sung Eun Kim, Jae Hyun Bae, Shinae Kang, Jong Chul Won, Min-Jeong Shin, Heung Yong Jin, Jenny Moon, Hokyou Lee, Hyeon Chang Kim, In-Kyung Jeong
Diabetes & Metabolism Journal.2023; 47(5): 632. CrossRef - 2023 Clinical Practice Guidelines for Diabetes
Min Kyong Moon
The Journal of Korean Diabetes.2023; 24(3): 120. CrossRef - 2023 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association
Jong Han Choi, Kyung Ae Lee, Joon Ho Moon, Suk Chon, Dae Jung Kim, Hyun Jin Kim, Nan Hee Kim, Ji A Seo, Mee Kyoung Kim, Jeong Hyun Lim, YoonJu Song, Ye Seul Yang, Jae Hyeon Kim, You-Bin Lee, Junghyun Noh, Kyu Yeon Hur, Jong Suk Park, Sang Youl Rhee, Hae J
Diabetes & Metabolism Journal.2023; 47(5): 575. CrossRef - Management of Dyslipidemia in Patients with Diabetes Mellitus
Kyung Ae Lee
The Journal of Korean Diabetes.2023; 24(3): 111. CrossRef - 2023 Clinical Practice Guidelines for Diabetes: Management of Cardiovascular Risk Factors
Ye Seul Yang
The Journal of Korean Diabetes.2023; 24(3): 135. CrossRef - Dyslipidemia Fact Sheet in South Korea, 2022
Eun-Sun Jin, Jee-Seon Shim, Sung Eun Kim, Jae Hyun Bae, Shinae Kang, Jong Chul Won, Min-Jeong Shin, Heung Yong Jin, Jenny Moon, Hokyou Lee, Hyeon Chang Kim, In-Kyung Jeong
Journal of Lipid and Atherosclerosis.2023; 12(3): 237. CrossRef
- Associations of omega-3 fatty acids vs. fenofibrate with adverse cardiovascular outcomes in people with metabolic syndrome: propensity matched cohort study
- Drug/Regimen
- New, Novel Lipid-Lowering Agents for Reducing Cardiovascular Risk: Beyond Statins
- Kyuho Kim, Henry N. Ginsberg, Sung Hee Choi
- Diabetes Metab J. 2022;46(4):517-532. Published online July 27, 2022
- DOI: https://doi.org/10.4093/dmj.2022.0198
- Correction in: Diabetes Metab J 2022;46(5):817
- 9,974 View
- 864 Download
- 25 Web of Science
- 25 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
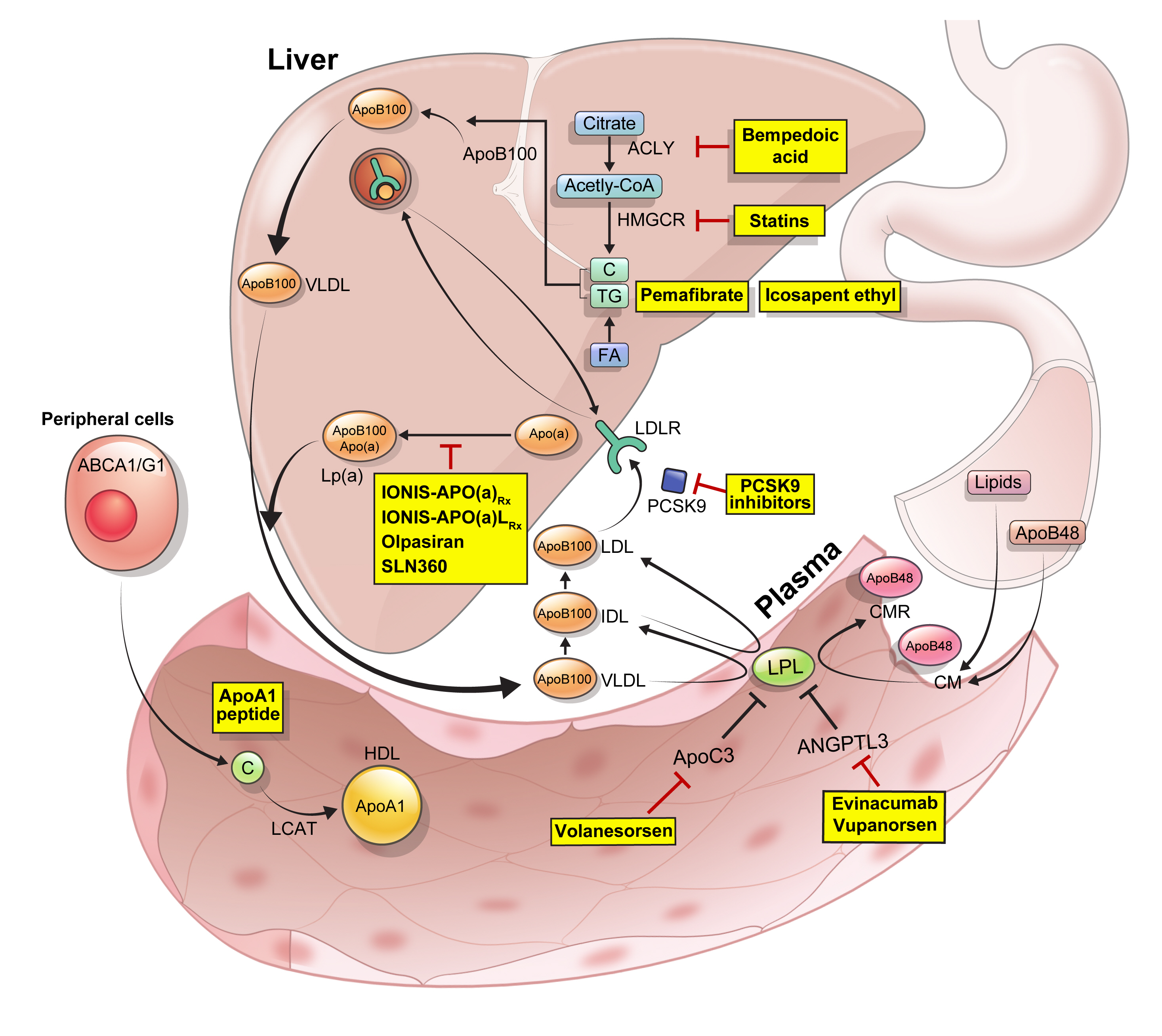
ePub - Statins are the cornerstone of the prevention and treatment of atherosclerotic cardiovascular disease (ASCVD). However, even under optimal statin therapy, a significant residual ASCVD risk remains. Therefore, there has been an unmet clinical need for novel lipid-lowering agents that can target low-density lipoprotein cholesterol (LDL-C) and other atherogenic particles. During the past decade, several drugs have been developed for the treatment of dyslipidemia. Inclisiran, a small interfering RNA that targets proprotein convertase subtilisin/kexin type 9 (PCSK9), shows comparable effects to that of PCSK9 monoclonal antibodies. Bempedoic acid, an ATP citrate lyase inhibitor, is a valuable treatment option for the patients with statin intolerance. Pemafibrate, the first selective peroxisome proliferator-activated receptor alpha modulator, showed a favorable benefit-risk balance in phase 2 trial, but the large clinical phase 3 trial (PROMINENT) was recently stopped for futility based on a late interim analysis. High dose icosapent ethyl, a modified eicosapentaenoic acid preparation, shows cardiovascular benefits. Evinacumab, an angiopoietin-like 3 (ANGPTL3) monoclonal antibody, reduces plasma LDL-C levels in patients with refractory hypercholesterolemia. Novel antisense oligonucleotides targeting apolipoprotein C3 (apoC3), ANGPTL3, and lipoprotein(a) have significantly attenuated the levels of their target molecules with beneficial effects on associated dyslipidemias. Apolipoprotein A1 (apoA1) is considered as a potential treatment to exploit the athero-protective effects of high-density lipoprotein cholesterol (HDL-C), but solid clinical evidence is necessary. In this review, we discuss the mode of action and clinical outcomes of these novel lipid-lowering agents beyond statins.
-
Citations
Citations to this article as recorded by- The role of adherence in patients with chronic diseases
Michel Burnier
European Journal of Internal Medicine.2024; 119: 1. CrossRef - Bempedoic acid: new evidence and recommendations on use
Kristina Paponja, Ivan Pećin, Željko Reiner, Maciej Banach
Current Opinion in Lipidology.2024; 35(1): 41. CrossRef - Genetic insights into repurposing statins for hyperthyroidism prevention: a drug-target Mendelian randomization study
Anqi Huang, Xinyi Wu, Jiaqi Lin, Chiju Wei, Wencan Xu
Frontiers in Endocrinology.2024;[Epub] CrossRef - Targeting host-specific metabolic pathways—opportunities and challenges for anti-infective therapy
Monika I. Konaklieva, Balbina J. Plotkin
Frontiers in Molecular Biosciences.2024;[Epub] CrossRef - Neutrophil Extracellular Traps (NETs) and Atherosclerosis: Does Hypolipidemic Treatment Have an Effect?
Petros Adamidis, Despoina Pantazi, Iraklis Moschonas, Evangelos Liberopoulos, Alexandros Tselepis
Journal of Cardiovascular Development and Disease.2024; 11(3): 72. CrossRef - Modulating effects of crocin on lipids and lipoproteins: Mechanisms and potential benefits
Habib Yaribeygi, Mina Maleki, Farin Rashid-Farrokhi, Payman Raise Abdullahi, Mohammad Amin Hemmati, Tannaz Jamialahmadi, Amirhossein Sahebkar
Heliyon.2024; 10(7): e28837. CrossRef - Assessing the Benefits of Lifestyle Influences on Cardiovascu-lar Health After Acute Coronary Syndrome
Marius Rus, Claudia Elena Stanis, Paula Marian, Lilliana Oana Pobirci, Loredana Ioana Banszki, Veronica Huplea, Gheorghe Adrian Osiceanu, Bianca-Maria Pop, Gabriela Dogaru, Felicia Liana Andronie-Cioara
Balneo and PRM Research Journal.2024; 15(Vol.15, no): 660. CrossRef - Liver cancer cells as the model for developing liver-targeted RNAi therapeutics
Beibei Hou, Linhui Qin, Linfeng Huang
Biochemical and Biophysical Research Communications.2023; 644: 85. CrossRef - Insights into Causal Cardiovascular Risk Factors from Mendelian Randomization
C. M. Schooling, J. V. Zhao
Current Cardiology Reports.2023; 25(2): 67. CrossRef - Secoisolariciresinol diglucoside and anethole ameliorate lipid abnormalities, oxidative injury, hypercholesterolemia, heart, and liver conditions
Sana Noreen, Habib‐ur Rehman, Tabussam Tufail, Huma Badar Ul Ain, Chinaza Godswill Awuchi
Food Science & Nutrition.2023; 11(6): 2620. CrossRef - Colesterol remanente, riesgo vascular y prevención de la arteriosclerosis
Xavier Pintó, Marta Fanlo, Virginia Esteve, Jesús Millán, Agustín Blanco, Mariano Blasco, José Luís Díaz Díaz, Ángel Díaz Rodríguez, Alipio Mangas, Vicente Pascual, Juan Pedro Botet, Pablo Pérez Martínez
Clínica e Investigación en Arteriosclerosis.2023; 35(4): 206. CrossRef - Evolving Management of Low‐Density Lipoprotein Cholesterol: A Personalized Approach to Preventing Atherosclerotic Cardiovascular Disease Across the Risk Continuum
Michael J. Wilkinson, Norman E. Lepor, Erin D. Michos
Journal of the American Heart Association.2023;[Epub] CrossRef - The cell origins of foam cell and lipid metabolism regulated by mechanical stress in atherosclerosis
Zhi Ouyang, Jian Zhong, Junyi Shen, Ye Zeng
Frontiers in Physiology.2023;[Epub] CrossRef - Triglyceride-Rich Lipoprotein Metabolism: Key Regulators of Their Flux
Alejandro Gugliucci
Journal of Clinical Medicine.2023; 12(13): 4399. CrossRef - Remnant cholesterol, vascular risk, and prevention of atherosclerosis
Xavier Pintó, Marta Fanlo, Virginia Esteve, Jesús Millán
Clínica e Investigación en Arteriosclerosis (English Edition).2023; 35(4): 206. CrossRef - Antibiotics and Lipid-Modifying Agents: Potential Drug–Drug Interactions and Their Clinical Implications
Marios Spanakis, Danny Alon-Ellenbogen, Petros Ioannou, Nikolaos Spernovasilis
Pharmacy.2023; 11(4): 130. CrossRef - Advances in Treatment of Dyslipidemia
Jill Dybiec, Wiktoria Baran, Bartłomiej Dąbek, Piotr Fularski, Ewelina Młynarska, Ewa Radzioch, Jacek Rysz, Beata Franczyk
International Journal of Molecular Sciences.2023; 24(17): 13288. CrossRef - Peroxisome Proliferator-Activated Receptor α in Lipoprotein Metabolism and Atherosclerotic Cardiovascular Disease
Elena Valeria Fuior, Evangelia Zvintzou, Theodosios Filippatos, Katerina Giannatou, Victoria Mparnia, Maya Simionescu, Anca Violeta Gafencu, Kyriakos E. Kypreos
Biomedicines.2023; 11(10): 2696. CrossRef - Preparation, characterization and in vivo pharmacokinetic study of ginsenoside Rb1-PLGA nanoparticles
Lixin Du, Huiling Lu, Yifei Xiao, Zhihua Guo, Ya Li
Scientific Reports.2023;[Epub] CrossRef - Dysregulation of Cholesterol Homeostasis in Ovarian Cancer
Zahraa Qusairy, Anne Gangloff, Shuk On Annie Leung
Current Oncology.2023; 30(9): 8386. CrossRef - Riesgo residual. Conclusiones
Ángel Cequier, José Luis Zamorano
Revista Española de Cardiología Suplementos.2023; 23: 25. CrossRef - Causal effects of circulating lipids and lipid-lowering drugs on the risk of urinary stones: a Mendelian randomization study
Zilong Tan, Jing Hong, Aochuan Sun, Mengdi Ding, Jianwu Shen
Frontiers in Endocrinology.2023;[Epub] CrossRef - Bibliometric analysis of residual cardiovascular risk: trends and frontiers
Lin Wang, Sutong Wang, Chaoyuan Song, Yiding Yu, Yuehua Jiang, Yongcheng Wang, Xiao Li
Journal of Health, Population and Nutrition.2023;[Epub] CrossRef - Current Understanding on the Genetic Basis of Key Metabolic Disorders: A Review
Kenneth Francis Rodrigues, Wilson Thau Lym Yong, Md. Safiul Alam Bhuiyan, Shafiquzzaman Siddiquee, Muhammad Dawood Shah, Balu Alagar Venmathi Maran
Biology.2022; 11(9): 1308. CrossRef - Lipoprotein Lipase: Is It a Magic Target for the Treatment of Hypertriglyceridemia
Joon Ho Moon, Kyuho Kim, Sung Hee Choi
Endocrinology and Metabolism.2022; 37(4): 575. CrossRef
- The role of adherence in patients with chronic diseases
- Metabolic Risk/Epidemiology
- Current Status of Low-Density Lipoprotein Cholesterol Target Achievement in Patients with Type 2 Diabetes Mellitus in Korea Compared with Recent Guidelines
- Soo Jin Yun, In-Kyung Jeong, Jin-Hye Cha, Juneyoung Lee, Ho Chan Cho, Sung Hee Choi, SungWan Chun, Hyun Jeong Jeon, Ho-Cheol Kang, Sang Soo Kim, Seung-Hyun Ko, Gwanpyo Koh, Su Kyoung Kwon, Jae Hyuk Lee, Min Kyong Moon, Junghyun Noh, Cheol-Young Park, Sungrae Kim
- Diabetes Metab J. 2022;46(3):464-475. Published online March 3, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0088
- 6,957 View
- 347 Download
- 4 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
We evaluated the achievement of low-density lipoprotein cholesterol (LDL-C) targets in patients with type 2 diabetes mellitus (T2DM) according to up-to-date Korean Diabetes Association (KDA), European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS), and American Diabetes Association (ADA) guidelines.
Methods
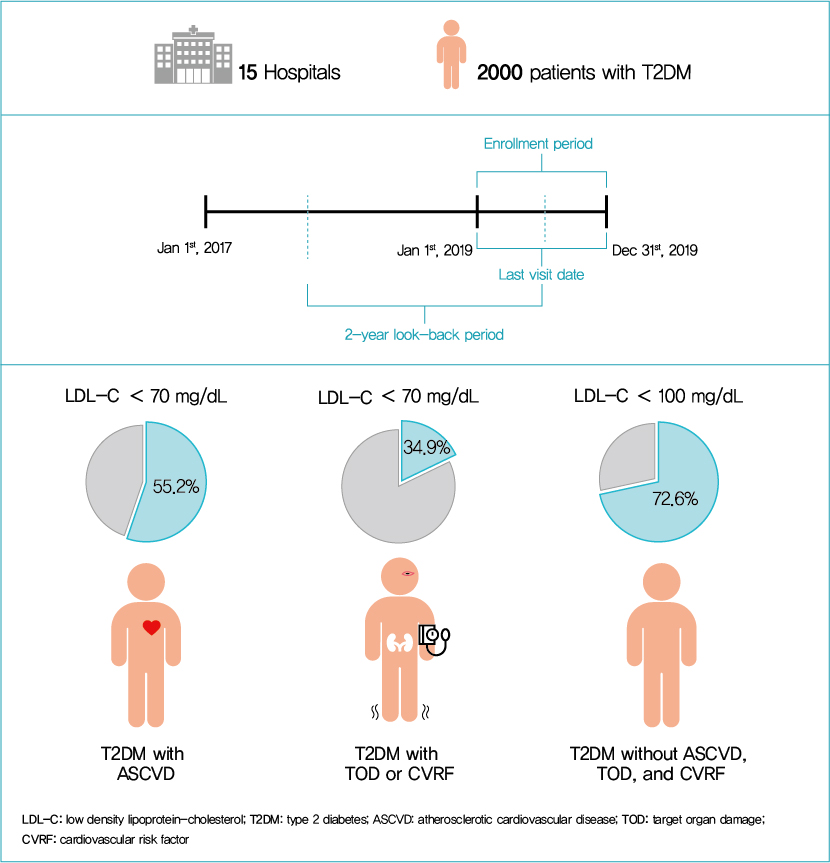
This retrospective cohort study collected electronic medical record data from patients with T2DM (≥20 years) managed by endocrinologists from 15 hospitals in Korea (January to December 2019). Patients were categorized according to guidelines to assess LDL-C target achievement. KDA (2019): Very High-I (atherosclerotic cardiovascular disease [ASCVD]) <70 mg/dL; Very High-II (target organ damage [TOD], or cardiovascular risk factors [CVRFs]) <70 mg/dL; high (others) <100 mg/dL. ESC/EAS (2019): Very High-I (ASCVD): <55 mg/dL; Very High-II (TOD or ≥3-CVRF) <55 mg/dL; high (diabetes ≥10 years without TOD plus any CVRF) <70 mg/dL; moderate (diabetes <10 years without CVRF) <100 mg/dL. ADA (2019): Very High-I (ASCVD); Very High-II (age ≥40+ TOD, or any CVRF), for high intensity statin or statin combined with ezetimibe.
Results
Among 2,000 T2DM patients (mean age 62.6 years; male 55.9%; mean glycosylated hemoglobin 7.2%) ASCVD prevalence was 24.7%. Of 1,455 (72.8%) patients treated with statins, 73.9% received monotherapy. According to KDA guidelines, LDL-C target achievement rates were 55.2% in Very High-I and 34.9% in Very High-II patients. With ESC/EAS guidelines, target attainment rates were 26.6% in Very High-I, 15.7% in Very High-II, and 25.9% in high risk patients. Based on ADA guidelines, most patients (78.9%) were very-high risk; however, only 15.5% received high-intensity statin or combination therapy.
Conclusion
According to current dyslipidemia management guidelines, LDL-C goal achievement remains suboptimal in Korean patients with T2DM. -
Citations
Citations to this article as recorded by- Risk factor control and cardiovascular events in patients with type 2 diabetes mellitus
Do Kyeong Song, Young Sun Hong, Yeon-Ah Sung, Hyejin Lee, Hidetaka Hamasaki
PLOS ONE.2024; 19(2): e0299035. CrossRef - Distinct effects of rosuvastatin and rosuvastatin/ezetimibe on senescence markers of CD8+ T cells in patients with type 2 diabetes mellitus: a randomized controlled trial
Sang-Hyeon Ju, Joung Youl Lim, Minchul Song, Ji Min Kim, Yea Eun Kang, Hyon-Seung Yi, Kyong Hye Joung, Ju Hee Lee, Hyun Jin Kim, Bon Jeong Ku
Frontiers in Endocrinology.2024;[Epub] CrossRef - Lipid Management in Korean People With Type 2 Diabetes Mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement
Ye Seul Yang, Hack-Lyoung Kim, Sang-Hyun Kim, Min Kyong Moon
Journal of Lipid and Atherosclerosis.2023; 12(1): 12. CrossRef - Lipid Management in Korean People with Type 2 Diabetes Mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement
Ye Seul Yang, Hack-Lyoung Kim, Sang-Hyun Kim, Min Kyong Moon
Diabetes & Metabolism Journal.2023; 47(1): 1. CrossRef - Management of Dyslipidemia in Patients with Diabetes Mellitus
Kyung Ae Lee
The Journal of Korean Diabetes.2023; 24(3): 111. CrossRef - Association between carotid atherosclerosis and presence of intracranial atherosclerosis using three-dimensional high-resolution vessel wall magnetic resonance imaging in asymptomatic patients with type 2 diabetes
Ji Eun Jun, You-Cheol Hwang, Kyu Jeong Ahn, Ho Yeon Chung, Geon-Ho Jahng, Soonchan Park, In-Kyung Jeong, Chang-Woo Ryu
Diabetes Research and Clinical Practice.2022; 191: 110067. CrossRef
- Risk factor control and cardiovascular events in patients with type 2 diabetes mellitus
- Metabolic Risk/Epidemiology
- Computed Tomography-Derived Myosteatosis and Metabolic Disorders
- Iva Miljkovic, Chantal A. Vella, Matthew Allison
- Diabetes Metab J. 2021;45(4):482-491. Published online July 30, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0277
- 6,222 View
- 236 Download
- 41 Web of Science
- 44 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub 
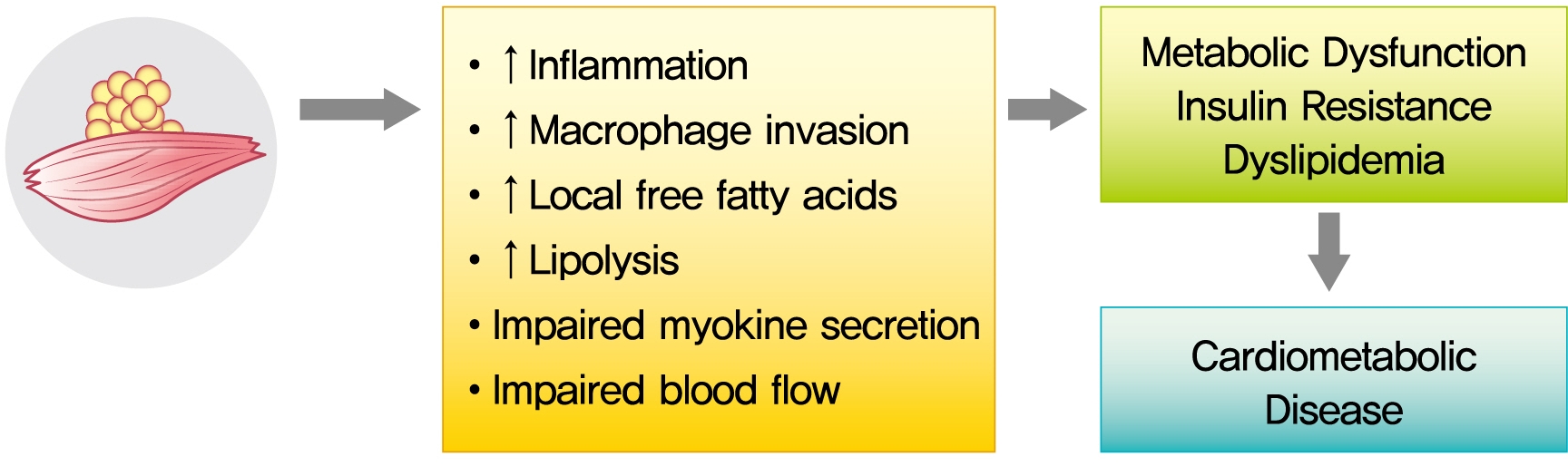
- The role of ectopic adipose tissue infiltration into skeletal muscle (i.e., myosteatosis) for metabolic disorders has received considerable and increasing attention in the last 10 years. The purpose of this review was to evaluate and summarize existing studies focusing on computed tomography (CT)-derived measures of myosteatosis and metabolic disorders. There is consistent evidence that CT-derived myosteatosis contributes to dysglycemia, insulin resistance, type 2 diabetes mellitus, and inflammation, and, to some extent, dyslipidemia, independent of general obesity, visceral fat, and other relevant risk factors, suggesting that it may serve as a tool for metabolic risk prediction. Identification of which muscles should be examined, and the standardized CT protocols to be employed, are necessary to enhance the applicability of findings from epidemiologic studies of myosteatosis. Additional and longer longitudinal studies are necessary to confirm a role of myosteatosis in the development of type 2 diabetes mellitus, and examine these associations in a variety of muscles across multiple race/ethnic populations. Given the emerging role of myosteatosis in metabolic health, well-designed intervention studies are needed to investigate relevant lifestyle and pharmaceutical approaches.
-
Citations
Citations to this article as recorded by- Association of Muscle Fat Content and Muscle Mass With Impaired Lung Function in Young Adults With Obesity: Evaluation With MRI
Xin Yu, Yan-Hao Huang, You-Zhen Feng, Zhong-Yuan Cheng, Cun-Chuan Wang, Xiang-Ran Cai
Academic Radiology.2024; 31(1): 9. CrossRef - Skeletal muscle alterations indicate poor prognosis in cirrhotic patients: a multicenter cohort study in China
Xin Zeng, Zhi-Wen Shi, Jia-Jun Yu, Li-Fen Wang, Chun-Yan Sun, Yuan-Yuan Luo, Pei-Mei Shi, Yong Lin, Yue-Xiang Chen, Jia Guo, Chun-Qing Zhang, Wei-Fen Xie
Hepatology International.2024; 18(2): 673. CrossRef - Subtype-specific Body Composition and Metabolic Risk in Patients With Primary Aldosteronism
Seung Shin Park, Chang Ho Ahn, Sang Wan Kim, Ji Won Yoon, Jung Hee Kim
The Journal of Clinical Endocrinology & Metabolism.2024; 109(2): e788. CrossRef - Myosteatosis as a novel predictor of new‐onset diabetes mellitus after kidney transplantation
Takahito Wakamiya, Takuya Fujimoto, Takahito Endo, Shun Nishioka, Naoki Yokoyama, Shimpei Yamashita, Kazuro Kikkawa, Yoji Hyodo, Takeshi Ishimura, Yasuo Kohjimoto, Isao Hara, Masato Fujisawa
International Journal of Urology.2024; 31(1): 39. CrossRef - Predictors of visceral and subcutaneous adipose tissue and muscle density: The ShapeUp! Kids study
Gertraud Maskarinec, Yurii Shvetsov, Michael C. Wong, Devon Cataldi, Jonathan Bennett, Andrea K. Garber, Steven D. Buchthal, Steven B. Heymsfield, John A. Shepherd
Nutrition, Metabolism and Cardiovascular Diseases.2024; 34(3): 799. CrossRef - Association of daily carbohydrate intake with intermuscular adipose tissue in Korean individuals with obesity: a cross-sectional study
Ha-Neul Choi, Young-Seol Kim, Jung-Eun Yim
Nutrition Research and Practice.2024; 18(1): 78. CrossRef - Myosteatosis is associated with poor survival after kidney transplantation: a large retrospective cohort validation
Jie Chen, Yue Li, Chengjie Li, Turun Song
Abdominal Radiology.2024; 49(4): 1210. CrossRef - Regenerative rehabilitation measures to restore tissue function after arsenic exposure
Adam A. Jasper, Kush H. Shah, Helmet Karim, Swathi Gujral, Iva Miljkovic, Caterina Rosano, Aaron Barchowsky, Amrita Sahu
Current Opinion in Biomedical Engineering.2024; 30: 100529. CrossRef - Impact of CFTR modulator therapy on body composition as assessed by thoracic computed tomography: A follow-up study
Víctor Navas-Moreno, Fernando Sebastian-Valles, Víctor Rodríguez-Laval, Carolina Knott-Torcal, Mónica Marazuela, Nuria Sánchez de la Blanca, Jose Alfonso Arranz Martín, Rosa María Girón, Miguel Antonio Sampedro-Núñez
Nutrition.2024; 123: 112425. CrossRef - Myosteatosis predicts postoperative complications and long‐term survival in robotic gastrectomy for gastric cancer: A propensity score analysis
Pingan Ding, Jiaxiang Wu, Haotian Wu, Tongkun Li, Jiaxuan Yang, Li Yang, Honghai Guo, Yuan Tian, Peigang Yang, Lingjiao Meng, Qun Zhao
European Journal of Clinical Investigation.2024;[Epub] CrossRef - A multifaceted and inclusive methodology for the detection of sarcopenia in patients undergoing bariatric surgery: an in-depth analysis of current evidence
Eunhye Seo, Yeongkeun Kwon, Ahmad ALRomi, Mohannad Eledreesi, Sungsoo Park
Reviews in Endocrine and Metabolic Disorders.2024;[Epub] CrossRef - Body Composition at CT and Risk of Future Disease
Michael A. Ohliger
Radiology.2023;[Epub] CrossRef - Association between hypertension and myosteatosis evaluated by abdominal computed tomography
Han Na Jung, Yun Kyung Cho, Hwi Seung Kim, Eun Hee Kim, Min Jung Lee, Woo Je Lee, Hong-Kyu Kim, Chang Hee Jung
Hypertension Research.2023; 46(4): 845. CrossRef - Muscle fat infiltration in chronic kidney disease: a marker related to muscle quality, muscle strength and sarcopenia
Carla Maria Avesani, Aline Miroski de Abreu, Heitor S. Ribeiro, Torkel B. Brismar, Peter Stenvinkel, Alice Sabatino, Bengt Lindholm
Journal of Nephrology.2023; 36(3): 895. CrossRef - Myosteatosis: a potential missing link between hypertension and metabolic disorder in the Asian population
Minyoung Lee, Sungha Park
Hypertension Research.2023; 46(6): 1603. CrossRef - Interplay of skeletal muscle and adipose tissue: sarcopenic obesity
Min Jeong Park, Kyung Mook Choi
Metabolism.2023; 144: 155577. CrossRef - Association between sarcopenic obesity and poor muscle quality based on muscle quality map and abdominal computed tomography
Yun Kyung Cho, Han Na Jung, Eun Hee Kim, Min Jung Lee, Joong‐Yeol Park, Woo Je Lee, Hong‐Kyu Kim, Chang Hee Jung
Obesity.2023; 31(6): 1547. CrossRef - Chest CT opportunistic biomarkers for phenotyping high-risk COVID-19 patients: a retrospective multicentre study
Anna Palmisano, Chiara Gnasso, Alberto Cereda, Davide Vignale, Riccardo Leone, Valeria Nicoletti, Simone Barbieri, Marco Toselli, Francesco Giannini, Marco Loffi, Gianluigi Patelli, Alberto Monello, Gianmarco Iannopollo, Davide Ippolito, Elisabetta Maria
European Radiology.2023; 33(11): 7756. CrossRef - Early menopause and premature ovarian insufficiency may increase the risk of sarcopenia: A systematic review and meta-analysis
Efstathios Divaris, Panagiotis Anagnostis, Nifon K. Gkekas, Evangelia Kouidi, Dimitrios G. Goulis
Maturitas.2023; 175: 107782. CrossRef - The Important Role of Intermuscular Adipose Tissue on Metabolic Changes Interconnecting Obesity, Ageing and Exercise: A Systematic Review
I Gusti Putu Suka Aryana, Ivana Beatrice Paulus, Sanjay Kalra, Dian Daniella, Raden Ayu Tuty Kuswardhani, Ketut Suastika, Sony Wibisono
European Endocrinology.2023; 19(1): 54. CrossRef - Increase in skeletal muscular adiposity and cognitive decline in a biracial cohort of older men and women
Caterina Rosano, Anne Newman, Adam Santanasto, Xiaonan Zhu, Bret Goodpaster, Iva Miljkovic
Journal of the American Geriatrics Society.2023; 71(9): 2759. CrossRef - Myosteatosis and bone marrow adiposity are not associated among postmenopausal women with fragility fractures
Sammy Badr, Héloïse Dapvril, Daniela Lombardo, Huda Khizindar, Claire Martin, Bernard Cortet, Anne Cotten, Julien Paccou
Frontiers in Endocrinology.2023;[Epub] CrossRef - Relationship between trunk intramuscular adipose tissue content and prevalence of metabolic syndrome in middle-aged Japanese men
Noriko I. Tanaka, Masataka Suwa, Hisashi Maeda, Aya Tomita, Takayuki Imoto, Hiroshi Akima
Nutrition.2023; 113: 112083. CrossRef - Sarcopenic obesity and its relation with muscle quality and mortality in patients on chronic hemodialysis
Alice Sabatino, Carla Maria Avesani, Giuseppe Regolisti, Marianna Adinolfi, Giuseppe Benigno, Marco Delsante, Enrico Fiaccadori, Ilaria Gandolfini
Clinical Nutrition.2023; 42(8): 1359. CrossRef - Skeletal muscle adiposity is a novel risk factor for poor cognition in African Caribbean women
Adrianna I. Acevedo‐Fontánez, Ryan K. Cvejkus, Joseph M. Zmuda, Allison L. Kuipers, Emma Barinas‐Mitchell, Akira Sekikawa, Victor Wheeler, Caterina Rosano, Iva Miljkovic
Obesity.2023; 31(9): 2398. CrossRef - Obesity, Sarcopenia and Myosteatosis: Impact on Clinical Outcomes in the Operative Management of Crohn’s Disease
Mark Donnelly, Dorothee Driever, Éanna J Ryan, Jessie A Elliott, John Finnegan, Deirdre McNamara, Ian Murphy, Kevin C Conlon, Paul C Neary, Dara O Kavanagh, James M O’Riordan
Inflammatory Bowel Diseases.2023;[Epub] CrossRef - Meld-sarcopenia score and skeletal muscle density predicts short-term readmission of patients with hepatic encephalopathy
Shuo Yang, Lin Zhang, Qian Jin, Jian Wang, Danli Ma, Jie Gao, Rui Huang
European Journal of Radiology.2023; 169: 111178. CrossRef - Muscle Fat Content Is Associated with Nonalcoholic Fatty Liver Disease and Liver Fibrosis in Chinese Adults
W. Guo, X. Zhao, D. Cheng, X. Liang, M. Miao, X. Li, J. Lu, N. Xu, Shuang Hu, Qun Zhang
The Journal of nutrition, health and aging.2023; 27(11): 960. CrossRef - Association between relative muscle strength and hypertension in middle-aged and older Chinese adults
Jin-hua Luo, Tu-ming Zhang, Lin-lin Yang, Yu-ying Cai, Yu Yang
BMC Public Health.2023;[Epub] CrossRef - Dynapenic Abdominal Obesity as a Risk Factor for Metabolic Syndrome in Individual 50 Years of Age or Older: English Longitudinal Study of Ageing
P.C. Ramírez, R. de Oliveira Máximo, D. Capra de Oliveira, A.F. de Souza, M. Marques Luiz, M. L. Bicigo Delinocente, A. Steptoe, C. de Oliveira, Tiago da Silva Alexandre
The Journal of nutrition, health and aging.2023; 27(12): 1188. CrossRef - Editorial Comment to Myosteatosis as a novel predictor of urinary incontinence after robot‐assisted radical prostatectomy
Nobuhiro Haga, Naotaka Gunge, Hiroshi Matsuzaki, Yu Okabe, Takeshi Miyazaki
International Journal of Urology.2022; 29(1): 40. CrossRef - Ammonia and the Muscle: An Emerging Point of View on Hepatic Encephalopathy
Simone Di Cola, Silvia Nardelli, Lorenzo Ridola, Stefania Gioia, Oliviero Riggio, Manuela Merli
Journal of Clinical Medicine.2022; 11(3): 611. CrossRef - Single skeletal muscle fiber mechanical properties: a muscle quality biomarker of human aging
Jae-Young Lim, Walter R. Frontera
European Journal of Applied Physiology.2022; 122(6): 1383. CrossRef - Sarcopenia in Patients with Cirrhosis after Transjugular Intrahepatic Portosystemic Shunt Placement
Jiacheng Liu, Jinqiang Ma, Chongtu Yang, Manman Chen, Qin Shi, Chen Zhou, Songjiang Huang, Yang Chen, Yingliang Wang, Tongqiang Li, Bin Xiong
Radiology.2022; 303(3): 711. CrossRef - Myosteatosis Significantly Predicts Persistent Dyspnea and Mobility Problems in COVID-19 Survivors
Rebecca De Lorenzo, Anna Palmisano, Antonio Esposito, Chiara Gnasso, Valeria Nicoletti, Riccardo Leone, Davide Vignale, Elisabetta Falbo, Marica Ferrante, Marta Cilla, Cristiano Magnaghi, Sabina Martinenghi, Giordano Vitali, Alessio Molfino, Patrizia Rove
Frontiers in Nutrition.2022;[Epub] CrossRef - Prognostic value of myosteatosis in patients with lung cancer: a systematic review and meta-analysis
Shaofang Feng, Huiwen Mu, Rong Hou, Yunxin Liu, Jianjun Zou, Zheng Zhao, Yubing Zhu
International Journal of Clinical Oncology.2022; 27(7): 1127. CrossRef - Muscle Fat Content Is Strongly Associated With Hyperuricemia: A Cross-Sectional Study in Chinese Adults
Ningxin Chen, Tingting Han, Hongxia Liu, Jie Cao, Wenwen Liu, Didi Zuo, Ting Zhang, Xiucai Lan, Xian Jin, Yurong Weng, Yaomin Hu
Frontiers in Endocrinology.2022;[Epub] CrossRef - Advances in muscle health and nutrition: A toolkit for healthcare professionals
Carla M. Prado, Francesco Landi, Samuel T.H. Chew, Philip J. Atherton, Jeroen Molinger, Tobias Ruck, Maria Cristina Gonzalez
Clinical Nutrition.2022; 41(10): 2244. CrossRef - Factors related to trunk intramuscular adipose tissue content – A comparison of younger and older men
Funa Kitagawa, Madoka Ogawa, Akito Yoshiko, Yoshiharu Oshida, Teruhiko Koike, Hiroshi Akima, Noriko I. Tanaka
Experimental Gerontology.2022; 168: 111922. CrossRef - Association of myosteatosis with various body composition abnormalities and longer length of hospitalization in patients with decompensated cirrhosis
Xiaoyu Wang, Mingyu Sun, Yifan Li, Gaoyue Guo, Wanting Yang, Lihong Mao, Zihan Yu, Yangyang Hui, Xiaofei Fan, Binxin Cui, Kui Jiang, Chao Sun
Frontiers in Nutrition.2022;[Epub] CrossRef - Peripheral bone structure, geometry, and strength and muscle density as derived from peripheral quantitative computed tomography and mortality among rural south Indian older adults
Guru Rajesh Jammy, Robert M. Boudreau, Iva Miljkovic, Pawan Kumar Sharma, Sudhakar Pesara Reddy, Susan L. Greenspan, Anne B. Newman, Jane A. Cauley, Bert B. Little
PLOS Global Public Health.2022; 2(10): e0000333. CrossRef - Muscle fat contents rather than muscle mass determines nonalcoholic steatohepatitis and liver fibrosis in patients with severe obesity
Eugene Han, Mi Kyung Kim, Hye Won Lee, Seungwan Ryu, Hye Soon Kim, Byoung Kuk Jang, Youngsung Suh
Obesity.2022; 30(12): 2440. CrossRef - Sex- and region-specific associations of skeletal muscle mass with metabolic dysfunction-associated fatty liver disease
Pei Xiao, Pu Liang, Panjun Gao, Jinyi Wu
Frontiers in Endocrinology.2022;[Epub] CrossRef - Imaging based body composition profiling and outcomes after oncologic liver surgery
Lorenzo Bernardi, Raffaello Roesel, Filippo Vagelli, Pietro Majno-Hurst, Alessandra Cristaudi
Frontiers in Oncology.2022;[Epub] CrossRef
- Association of Muscle Fat Content and Muscle Mass With Impaired Lung Function in Young Adults With Obesity: Evaluation With MRI
- Drug/Regimen
- Fibrates Revisited: Potential Role in Cardiovascular Risk Reduction
- Nam Hoon Kim, Sin Gon Kim
- Diabetes Metab J. 2020;44(2):213-221. Published online April 23, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0001
- 7,660 View
- 310 Download
- 41 Web of Science
- 42 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Fibrates, peroxisome proliferator-activated receptor-α agonists, are potent lipid-modifying drugs. Their main effects are reduction of triglycerides and increase in high-density lipoprotein levels. Several randomized controlled trials have not demonstrated their benefits on cardiovascular risk reduction, especially as an “add on” to statin therapy. However, subsequent analyses by major clinical trials, meta-analyses, and real-world evidence have proposed their potential in specific patient populations with atherogenic dyslipidemia and metabolic syndrome. Here, we have reviewed and discussed the accumulated data on fibrates to understand their current status in cardiovascular risk management.
-
Citations
Citations to this article as recorded by- Associations of omega-3 fatty acids vs. fenofibrate with adverse cardiovascular outcomes in people with metabolic syndrome: propensity matched cohort study
Nam Hoon Kim, Ji Yoon Kim, Jimi Choi, Sin Gon Kim
European Heart Journal - Cardiovascular Pharmacotherapy.2024; 10(2): 118. CrossRef - Role of PPARα in inflammatory response of C2C12 myotubes
Yuki Shimizu, Keiko Hamada, Tingting Guo, Chie Hasegawa, Yusuke Kuga, Katsushi Takeda, Takashi Yagi, Hiroyuki Koyama, Hiroshi Takagi, Daisuke Aotani, Hiromi Kataoka, Tomohiro Tanaka
Biochemical and Biophysical Research Communications.2024; 694: 149413. CrossRef - Obicetrapib: Reversing the Tide of CETP Inhibitor Disappointments
John J. P. Kastelein, Andrew Hsieh, Mary R. Dicklin, Marc Ditmarsch, Michael H. Davidson
Current Atherosclerosis Reports.2024; 26(2): 35. CrossRef - Metabolic Flexibility of the Heart: The Role of Fatty Acid Metabolism in Health, Heart Failure, and Cardiometabolic Diseases
Virginia Actis Dato, Stephan Lange, Yoshitake Cho
International Journal of Molecular Sciences.2024; 25(2): 1211. CrossRef - ApoB100 and Atherosclerosis: What’s New in the 21st Century?
Dimitris Kounatidis, Natalia G. Vallianou, Aikaterini Poulaki, Angelos Evangelopoulos, Fotis Panagopoulos, Theodora Stratigou, Eleni Geladari, Irene Karampela, Maria Dalamaga
Metabolites.2024; 14(2): 123. CrossRef - Follistatin-like 1 (FSTL1) levels as potential early biomarker of cardiovascular disease in a Mexican population
N. Ponce-Ruíz, J. F. Herrera-Moreno, A. E. Rojas-García, B. S. Barrón-Vivanco, C. A. González-Arias, Y. Y. Bernal-Hernández, L. Ortega-Cervantes, J. Ponce-Gallegos, J. A. Hernández-Nolasco, I. M. Medina-Díaz
Heart and Vessels.2024;[Epub] CrossRef - Role of Fenofibrate Use in Dyslipidemia and Related Comorbidities in the Asian Population: A Narrative Review
Chaicharn Deerochanawong, Sin Gon Kim, Yu-Cheng Chang
Diabetes & Metabolism Journal.2024; 48(2): 184. CrossRef - Coenzyme Q10 in atherosclerosis
Minjun Liao, Xueke He, Yangyang Zhou, Weiqiang Peng, Xiao-Mei Zhao, Miao Jiang
European Journal of Pharmacology.2024; 970: 176481. CrossRef - Onion Polyphenols as Multi-Target-Directed Ligands in MASLD: A Preliminary Molecular Docking Study
Maria Rosaria Paravati, Anna Caterina Procopio, Maja Milanović, Giuseppe Guido Maria Scarlata, Nataša Milošević, Maja Ružić, Nataša Milić, Ludovico Abenavoli
Nutrients.2024; 16(8): 1226. CrossRef - Present and Future of Dyslipidaemia Treatment—A Review
Iveta Merćep, Andro Vujević, Dominik Strikić, Ivana Radman, Ivan Pećin, Željko Reiner
Journal of Clinical Medicine.2023; 12(18): 5839. CrossRef - VLDL receptor gene therapy for reducing atherogenic lipoproteins
Ronald M. Krauss, Jonathan T. Lu, Joseph J. Higgins, Cathryn M. Clary, Ray Tabibiazar
Molecular Metabolism.2023; 69: 101685. CrossRef - The emerging role of PPAR-alpha in breast cancer
Zhiwen Qian, Lingyan Chen, Jiayu Liu, Ying Jiang, Yan Zhang
Biomedicine & Pharmacotherapy.2023; 161: 114420. CrossRef - Molecular mechanisms and therapeutic perspectives of peroxisome proliferator‐activated receptor α agonists in cardiovascular health and disease
Yujie Pu, Chak Kwong Cheng, Hongsong Zhang, Jiang‐Yun Luo, Li Wang, Brian Tomlinson, Yu Huang
Medicinal Research Reviews.2023; 43(6): 2086. CrossRef - Macrophage angiotensin-converting enzyme reduces atherosclerosis by increasing peroxisome proliferator-activated receptor α and fundamentally changing lipid metabolism
DuoYao Cao, Zakir Khan, Xiaomo Li, Suguru Saito, Ellen A Bernstein, Aaron R Victor, Faizan Ahmed, Aoi O Hoshi, Luciana C Veiras, Tomohiro Shibata, Mingtian Che, Lei Cai, Michifumi Yamashita, Ryan E Temel, Jorge F Giani, Daniel J Luthringer, Ajit S Divakar
Cardiovascular Research.2023; 119(9): 1825. CrossRef - Rapid flow synthesis of fenofibrate via scalable flash chemistry with in-line Li recovery
Sanket A. Kawale, Dong-Chang Kang, Gwang-Noh Ahn, Amirreza Mottafegh, Ji-Ho Kang, Gi-Su Na, Dong-Pyo Kim
Chemical Engineering Journal.2023; 477: 147033. CrossRef - Effectiveness and Safety of Fenofibrate in Routine Treatment of Patients with Hypertriglyceridemia and Metabolic Syndrome
Marat V. Ezhov, Gregory P. Arutyunov
Diseases.2023; 11(4): 140. CrossRef - Development of New Genome Editing Tools for the Treatment of Hyperlipidemia
Giulio Preta
Cells.2023; 12(20): 2466. CrossRef - Exploring the hypolipidemic effects of bergenin from Saxifraga melanocentra Franch: mechanistic insights and potential for hyperlipidemia treatment
Li Zhang, Yingying Tong, Yan Fang, Jinjin Pei, Qilan Wang, Gang Li
Lipids in Health and Disease.2023;[Epub] CrossRef - Obesity and Dyslipidemia
Barbora Nussbaumerova, Hana Rosolova
Current Atherosclerosis Reports.2023; 25(12): 947. CrossRef - Bibliometric analysis of residual cardiovascular risk: trends and frontiers
Lin Wang, Sutong Wang, Chaoyuan Song, Yiding Yu, Yuehua Jiang, Yongcheng Wang, Xiao Li
Journal of Health, Population and Nutrition.2023;[Epub] CrossRef - Hypertriglyceridemia in Apoa5–/– mice results from reduced amounts of lipoprotein lipase in the capillary lumen
Ye Yang, Anne P. Beigneux, Wenxin Song, Le Phuong Nguyen, Hyesoo Jung, Yiping Tu, Thomas A. Weston, Caitlyn M. Tran, Katherine Xie, Rachel G. Yu, Anh P. Tran, Kazuya Miyashita, Katsuyuki Nakajima, Masami Murakami, Yan Q. Chen, Eugene Y. Zhen, Joonyoung R.
Journal of Clinical Investigation.2023;[Epub] CrossRef - Blood-Derived Lipid and Metabolite Biomarkers in Cardiovascular Research from Clinical Studies: A Recent Update
Dipali Kale, Amol Fatangare, Prasad Phapale, Albert Sickmann
Cells.2023; 12(24): 2796. CrossRef - Effective, disease-modifying, clinical approaches to patients with mild-to-moderate hypertriglyceridaemia
Gary F Lewis, Robert A Hegele
The Lancet Diabetes & Endocrinology.2022; 10(2): 142. CrossRef - Effects of Alirocumab on Triglyceride Metabolism: A Fat-Tolerance Test and Nuclear Magnetic Resonance Spectroscopy Study
Thomas Metzner, Deborah R. Leitner, Karin Mellitzer, Andrea Beck, Harald Sourij, Tatjana Stojakovic, Gernot Reishofer, Winfried März, Ulf Landmesser, Hubert Scharnagl, Hermann Toplak, Günther Silbernagel
Biomedicines.2022; 10(1): 193. CrossRef - Is there a role of lipid-lowering therapies in the management of fatty liver disease?
Ismini Tzanaki, Aris P Agouridis, Michael S Kostapanos
World Journal of Hepatology.2022; 14(1): 119. CrossRef - Therapeutic Strategies and Chemoprevention of Atherosclerosis: What Do We Know and Where Do We Go?
Ana Clara Aprotosoaie, Alexandru-Dan Costache, Irina-Iuliana Costache
Pharmaceutics.2022; 14(4): 722. CrossRef - The Overlooked Transformation Mechanisms of VLCFAs: Peroxisomal β-Oxidation
Qinyue Lu, Weicheng Zong, Mingyixing Zhang, Zhi Chen, Zhangping Yang
Agriculture.2022; 12(7): 947. CrossRef - Current Trends of Big Data Research Using the Korean National Health Information Database
Mee Kyoung Kim, Kyungdo Han, Seung-Hwan Lee
Diabetes & Metabolism Journal.2022; 46(4): 552. CrossRef - New, Novel Lipid-Lowering Agents for Reducing Cardiovascular Risk: Beyond Statins
Kyuho Kim, Henry N. Ginsberg, Sung Hee Choi
Diabetes & Metabolism Journal.2022; 46(4): 517. CrossRef - Novel Targets for a Combination of Mechanical Unloading with Pharmacotherapy in Advanced Heart Failure
Agata Jedrzejewska, Alicja Braczko, Ada Kawecka, Marcin Hellmann, Piotr Siondalski, Ewa Slominska, Barbara Kutryb-Zajac, Magdi H. Yacoub, Ryszard T. Smolenski
International Journal of Molecular Sciences.2022; 23(17): 9886. CrossRef - Lipoprotein Lipase: Is It a Magic Target for the Treatment of Hypertriglyceridemia
Joon Ho Moon, Kyuho Kim, Sung Hee Choi
Endocrinology and Metabolism.2022; 37(4): 575. CrossRef - Fenofibrate add-on to statin treatment is associated with low all-cause death and cardiovascular disease in the general population with high triglyceride levels
Kyung-Soo Kim, Sangmo Hong, Kyungdo Han, Cheol-Young Park
Metabolism.2022; 137: 155327. CrossRef - Alterations of HDL’s to piHDL’s Proteome in Patients with Chronic Inflammatory Diseases, and HDL-Targeted Therapies
Veronika Vyletelová, Mária Nováková, Ľudmila Pašková
Pharmaceuticals.2022; 15(10): 1278. CrossRef - Cardiovascular Risk Profile and Lipid Management in the Population-Based Cohort Study LATINO: 20 Years of Real-World Data
Cristina Gavina, Daniel Seabra Carvalho, Marisa Pardal, Marta Afonso-Silva, Diana Grangeia, Ricardo Jorge Dinis-Oliveira, Francisco Araújo, Tiago Taveira-Gomes
Journal of Clinical Medicine.2022; 11(22): 6825. CrossRef - New and emerging lipid-lowering therapy
James M Backes, Daniel E Hilleman
Future Cardiology.2021; 17(8): 1407. CrossRef - Systemic PFOS and PFOA exposure and disturbed lipid homeostasis in humans: what do we know and what not?
Styliani Fragki, Hubert Dirven, Tony Fletcher, Bettina Grasl-Kraupp, Kristine Bjerve Gützkow, Ron Hoogenboom, Sander Kersten, Birgitte Lindeman, Jochem Louisse, Ad Peijnenburg, Aldert H. Piersma, Hans M. G. Princen, Maria Uhl, Joost Westerhout, Marco J. Z
Critical Reviews in Toxicology.2021; 51(2): 141. CrossRef -
A network pharmacology analysis on drug‐like compounds from
Ganoderma lucidum
for alleviation of atherosclerosis
Ki Kwang Oh, Md. Adnan, Dong Ha Cho
Journal of Food Biochemistry.2021;[Epub] CrossRef - Efficacy and Safety of Fenofibrate-Statin Combination Therapy in Patients With Inadequately Controlled Triglyceride Levels Despite Previous Statin Monotherapy: A Multicenter, Randomized, Double-blind, Phase IV Study
Myung Soo Park, Jong-Chan Youn, Eung Ju Kim, Ki Hoon Han, Sang Hak Lee, Sung Hea Kim, Byung Jin Kim, Sung Uk Kwon, Kyu-Hyung Ryu
Clinical Therapeutics.2021; 43(10): 1735. CrossRef - Prevalence of and Factors Associated With the Prescription of Fibrates Among Patients Receiving Lipid-Lowering Drugs in Germany
Louis Jacob, Roger-Axel Greiner, Mark Luedde, Karel Kostev
Journal of Cardiovascular Pharmacology.2021; 78(6): 885. CrossRef - Challenging Issues in the Management of Cardiovascular Risk Factors in Diabetes During the COVID-19 Pandemic: A Review of Current Literature
Leili Rahimi, Mojtaba Malek, Faramarz Ismail-Beigi, Mohammad E. Khamseh
Advances in Therapy.2020; 37(8): 3450. CrossRef - Treatment With Gemfibrozil Prevents the Progression of Chronic Kidney Disease in Obese Dahl Salt-Sensitive Rats
Corbin A. Shields, Bibek Poudel, Kasi C. McPherson, Andrea K. Brown, Ubong S. Ekperikpe, Evan Browning, Lamari Sutton, Denise C. Cornelius, Jan M. Williams
Frontiers in Physiology.2020;[Epub] CrossRef - Oxidative Stress and Inflammation in Renal and Cardiovascular Complications of Diabetes
Amelia Charlton, Jessica Garzarella, Karin A. M. Jandeleit-Dahm, Jay C. Jha
Biology.2020; 10(1): 18. CrossRef
- Associations of omega-3 fatty acids vs. fenofibrate with adverse cardiovascular outcomes in people with metabolic syndrome: propensity matched cohort study
- Cardiovascular Risk/Epidemiology
- Pre-existing Depression among Newly Diagnosed Dyslipidemia Patients and Cardiovascular Disease Risk
- Jihoon Andrew Kim, Seulggie Choi, Daein Choi, Sang Min Park
- Diabetes Metab J. 2020;44(2):307-315. Published online November 1, 2019
- DOI: https://doi.org/10.4093/dmj.2019.0002
- 5,215 View
- 89 Download
- 9 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background Whether depression before diagnosis of dyslipidemia is associated with higher cardiovascular disease (CVD) risk among newly diagnosed dyslipidemia patients is yet unclear.
Methods The study population consisted of 72,235 newly diagnosed dyslipidemia patients during 2003 to 2012 from the National Health Insurance Service–Health Screening Cohort of South Korea. Newly diagnosed dyslipidemia patients were then detected for pre-existing depression within 3 years before dyslipidemia diagnosis. Starting from 2 years after the diagnosis date, patients were followed up for CVD until 2015. Adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) for CVD were calculated by Cox proportional hazards regression.
Results Compared to dyslipidemia patients without depression, those with depression had higher risk for CVD (aHR, 1.24; 95% CI, 1.09 to 1.41). Similarly, pre-existing depression was associated with increased risk for stroke (aHR, 1.27; 95% CI, 1.06 to 1.53). The risk for CVD among depressed dyslipidemia patients for high (aHR, 1.42; 95% CI, 1.06 to 1.90), medium (aHR, 1.17; 95% CI, 0.91 to 1.52), and low (aHR, 1.25; 95% CI, 1.05 to 1.50) statin compliance patients tended to be increased compared to patients without pre-existing dyslipidemia. The risk-elevating effect of depression on CVD tended to be preserved regardless of subgroups of smoking, alcohol consumption, physical activity, and body mass index.
Conclusion Dyslipidemia patients with pre-existing depression had increased risk for CVD. Future studies that determine CVD risk after management of depression among dyslipidemia patients are needed.
-
Citations
Citations to this article as recorded by- Mediating effect of depression on the association between cardiovascular disease and the risk of all‐cause mortality: NHANES in 2005−2018
Xinxin Ma, Huan Zhang, Yuan Tian, Yaping Wang, Ling Liu, Lei Wang
Clinical Cardiology.2023; 46(11): 1380. CrossRef - Associations of sleep duration, daytime napping, and snoring with depression in rural China: a cross-sectional study
Xueyao Zhang, Guangxiao Li, Chuning Shi, Yingxian Sun
BMC Public Health.2023;[Epub] CrossRef - Association between socioeconomic inequality and the global prevalence of anxiety and depressive disorders: an ecological study
Fatemeh Shahbazi, Marjan Shahbazi, Jalal Poorolajal
General Psychiatry.2022; 35(3): e100735. CrossRef - Impact of Alexithymia on the Lipid Profile in Major Depressed Individuals
Camille Point, Benjamin Wacquier, Marjorie Dosogne, Mohammed Al Faker, Hadrien Willame, Gwenolé Loas, Matthieu Hein, Philip W. Wertz
Journal of Lipids.2022; 2022: 1. CrossRef - Association of Depression With Cardiovascular Diseases
Zain I Warriach, Sruti Patel, Fatima Khan, Gerardo F Ferrer
Cureus.2022;[Epub] CrossRef - Fenofibrate add-on to statin treatment is associated with low all-cause death and cardiovascular disease in the general population with high triglyceride levels
Kyung-Soo Kim, Sangmo Hong, Kyungdo Han, Cheol-Young Park
Metabolism.2022; 137: 155327. CrossRef - Dyslipidemia prevalence and trends among adult mental disorder inpatients in Beijing, 2005–2018: A longitudinal observational study
Fude Yang, Qiuyue Ma, Botao Ma, Wenzhan Jing, Jue Liu, Moning Guo, Juan Li, Zhiren Wang, Min Liu
Asian Journal of Psychiatry.2021; 57: 102583. CrossRef - Non-HDL cholesterol level and depression among Canadian elderly—a cross-sectional analysis of the baseline data from the CLSA
Jian Liu, Surim Son, Mike Giancaterino, Chris P. Verschoor, Miya Narushima, David Moher
FACETS.2020; 5(1): 1006. CrossRef
- Mediating effect of depression on the association between cardiovascular disease and the risk of all‐cause mortality: NHANES in 2005−2018
- Epidemiology
- Association between Change in Alcohol Consumption and Metabolic Syndrome: Analysis from the Health Examinees Study
- Seulggie Choi, Kyuwoong Kim, Jong-Koo Lee, Ji-Yeob Choi, Aesun Shin, Sue Kyung Park, Daehee Kang, Sang Min Park
- Diabetes Metab J. 2019;43(5):615-626. Published online April 23, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0128
- 5,428 View
- 86 Download
- 14 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background The association between change in alcohol intake and metabolic syndrome is unclear.
Methods This retrospective cohort consisted of 41,368 males and females from the Health Examinees-GEM study. Participants were divided into non-drinkers (0.0 g/day), light drinkers (male: 0.1 to 19.9 g/day; female: 0.1 to 9.9 g/day), moderate drinkers (male: 20.0 to 39.9 g/day; female: 10.0 to 19.9 g/day), and heavy drinkers (male: ≥40.0 g/day; female: ≥20.0 g/day) for each of the initial and follow-up health examinations. Logistic regression analysis was used to determine the adjusted odds ratios (aORs) and 95% confidence intervals (CIs) for developing metabolic syndrome according to the change in alcohol consumption between the initial and follow-up health examinations. Adjusted mean values for the change in waist circumference, fasting serum glucose (FSG), blood pressure, triglycerides, and high density lipoprotein cholesterol (HDL-C) levels were determined according to the change in alcohol consumption by linear regression analysis.
Results Compared to persistent light drinkers, those who increased alcohol intake to heavy levels had elevated risk of metabolic syndrome (aOR, 1.45; 95% CI, 1.09 to 1.92). In contrast, heavy drinkers who became light drinkers had reduced risk of metabolic syndrome (aOR, 0.61; 95% CI, 0.44 to 0.84) compared to persistent heavy drinkers. Increased alcohol consumption was associated with elevated adjusted mean values for waist circumference, FSG, blood pressure, triglycerides, and HDL-C levels (all
P <0.05). Reduction in alcohol intake was associated with decreased waist circumference, FSG, blood pressure, triglycerides, and HDL-C levels among initial heavy drinkers (allP <0.05).Conclusion Heavy drinkers who reduce alcohol consumption could benefit from reduced risk of metabolic syndrome.
-
Citations
Citations to this article as recorded by- Inverse association between type 2 diabetes and hepatocellular carcinoma in East Asian populations
Jinlong Huo, Yaxuan Xu, Xingqi Chen, Jie Yu, Lijin Zhao
Frontiers in Endocrinology.2024;[Epub] CrossRef - Regulation Mechanism and Potential Value of Active Substances in Spices in Alcohol–Liver–Intestine Axis Health
Jianyu Huang, Tao Huang, Jinjun Li
International Journal of Molecular Sciences.2024; 25(7): 3728. CrossRef - Impact of green space and built environment on metabolic syndrome: A systematic review with meta-analysis
Muhammad Mainuddin Patwary, Mohammad Javad Zare Sakhvidi, Sadia Ashraf, Payam Dadvand, Matthew H.E.M. Browning, Md Ashraful Alam, Michelle L. Bell, Peter James, Thomas Astell-Burt
Science of The Total Environment.2024; 923: 170977. CrossRef - Causal effects of sleep traits on metabolic syndrome and its components: a Mendelian randomization study
Yongli Yang, Long Wen, Xuezhong Shi, Chaojun Yang, Jingwen Fan, Yi Zhang, Guibin Shen, Huiping Zhou, Xiaocan Jia
Sleep and Breathing.2024;[Epub] CrossRef - Use of biochemical markers for diabetes prevention in the new decade
Marie Chan Sun, Marie A. S. Landinaff, Ruben Thoplan
Physical Sciences Reviews.2023; 8(11): 3767. CrossRef - Alcohol consumption and metabolic syndrome: Clinical and epidemiological impact on liver disease
Fredrik Åberg, Christopher D. Byrne, Carlos J. Pirola, Ville Männistö, Silvia Sookoian
Journal of Hepatology.2023; 78(1): 191. CrossRef - Serum Nutritional Biomarkers and All-Cause and Cause-Specific Mortality in U.S. Adults with Metabolic Syndrome: The Results from National Health and Nutrition Examination Survey 2001–2006
Xinwei Peng, Jingjing Zhu, Henry S. Lynn, Xi Zhang
Nutrients.2023; 15(3): 553. CrossRef - Evaluation and Treatment of Obesity and Its Comorbidities: 2022 Update of Clinical Practice Guidelines for Obesity by the Korean Society for the Study of Obesity
Kyoung-Kon Kim, Ji-Hee Haam, Bom Taeck Kim, Eun Mi Kim, Jung Hwan Park, Sang Youl Rhee, Eonju Jeon, Eungu Kang, Ga Eun Nam, Hye Yeon Koo, Jeong-Hyun Lim, Jo-Eun Jeong, Jong-Hee Kim, Jong Won Kim, Jung Ha Park, Jun Hwa Hong, Sang Eok Lee, Se Hee Min, Seung
Journal of Obesity & Metabolic Syndrome.2023; 32(1): 1. CrossRef - Association between alcohol consumption and risk of hyperuricaemia among adults: a large cross-sectional study in Chongqing, China
Siyu Chen, Rui Ding, Xiaojun Tang, Liling Chen, Qinwen Luo, Meng Xiao, Xianbin Ding, Bin Peng
BMJ Open.2023; 13(12): e074697. CrossRef - Lifestyle Factors Associated with Metabolic Syndrome in Urban Cambodia
Miharu Tamaoki, Ikumi Honda, Keisuke Nakanishi, Maki Nakajima, Sophathya Cheam, Manabu Okawada, Hisataka Sakakibara
International Journal of Environmental Research and Public Health.2022; 19(17): 10481. CrossRef - Gender Differences of Health Behaviors in the Risk of Metabolic Syndrome for Middle-Aged Adults: A National Cross-Sectional Study in South Korea
Jaehee Yoon, Jeewuan Kim, Heesook Son
International Journal of Environmental Research and Public Health.2021; 18(7): 3699. CrossRef - Association between alcohol consumption and metabolic syndrome among Chinese adults
Yi Lin, Yan-Yan Ying, Si-Xuan Li, Si-Jia Wang, Qing-Hai Gong, Hui Li
Public Health Nutrition.2021; 24(14): 4582. CrossRef - Triglyceride-rich lipoprotein and LDL particle subfractions and their association with incident type 2 diabetes: the PREVEND study
Sara Sokooti, Jose L. Flores-Guerrero, Hiddo J. L. Heerspink, Margery A. Connelly, Stephan J. L. Bakker, Robin P. F. Dullaart
Cardiovascular Diabetology.2021;[Epub] CrossRef
- Inverse association between type 2 diabetes and hepatocellular carcinoma in East Asian populations
- Others
- Effect of Atorvastatin on Growth Differentiation Factor-15 in Patients with Type 2 Diabetes Mellitus and Dyslipidemia
- Ji Min Kim, Min Kyung Back, Hyon-Seung Yi, Kyong Hye Joung, Hyun Jin Kim, Bon Jeong Ku
- Diabetes Metab J. 2016;40(1):70-78. Published online February 19, 2016
- DOI: https://doi.org/10.4093/dmj.2016.40.1.70
- 4,177 View
- 33 Download
- 5 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Elevated serum levels of growth differentiation factor-15 (GDF-15) are associated with type 2 diabetes. Therefore, the effects of atorvastatin on metabolic parameters and GDF-15 levels in patients with type 2 diabetes and dyslipidemia were evaluated.
Methods In this prospective randomized trial from February 2013 to March 2014, 50 consecutive type 2 diabetic patients with a low density lipoprotein cholesterol (LDL-C) levels ≥100 mg/dL were enrolled. The patients were divided into two groups based on the amount of atorvastatin prescribed, 10 mg/day (
n =23) or 40 mg/day (n =27). The effect of atorvastatin on metabolic parameters, including lipid profiles and GDF-15 levels, at baseline and after 8 weeks of treatment were compared.Results The baseline metabolic parameters and GDF-15 levels were not significantly different between the two groups. After 8 weeks of treatment, the total cholesterol (TC) and LDL-C levels were significantly decreased in both groups. The mean changes in TC and LDL-C levels were more significant in the 40 mg atorvastatin group. The GDF-15 level was decreased in the 10 mg atorvastatin group, from 1,460.6±874.8 to 1,451.0±770.8 pg/mL, and was increased in the 40 mg atorvastatin group, from 1,271.6±801.0 to 1,341.4±855.2 pg/mL. However, the change in the GDF-15 level was not statistically significant in the 10 or 40 mg atorvastatin group (
P =0.665 andP =0.745, respectively).Conclusion The GDF-15 levels were not significantly changed after an 8-week treatment with atorvastatin in type 2 diabetic patients.
-
Citations
Citations to this article as recorded by- The relationship of Growth differentiation factor-15 with renal damage and dyslipidemia in non-albuminuric and albuminuric Type-2 Diabetes Mellitus
Hasan Esat Yücel, Bilal İlanbey
Medical Science and Discovery.2022; 9(6): 334. CrossRef - Comparative effectiveness of statins on non-high density lipoprotein cholesterol in people with diabetes and at risk of cardiovascular disease: systematic review and network meta-analysis
Alexander Hodkinson, Dialechti Tsimpida, Evangelos Kontopantelis, Martin K Rutter, Mamas A Mamas, Maria Panagioti
BMJ.2022; : e067731. CrossRef - The Cytokine Growth Differentiation Factor-15 and Skeletal Muscle Health: Portrait of an Emerging Widely Applicable Disease Biomarker
Boel De Paepe
International Journal of Molecular Sciences.2022; 23(21): 13180. CrossRef - Biomarkers of subclinical atherosclerosis in patients with psoriasis
Hannah Kaiser, Xing Wang, Amanda Kvist-Hansen, Martin Krakauer, Peter Michael Gørtz, Benjamin D. McCauley, Lone Skov, Christine Becker, Peter Riis Hansen
Scientific Reports.2021;[Epub] CrossRef - Growth differentiation factor-15 regulates oxLDL-induced lipid homeostasis and autophagy in human macrophages
Kathrin Ackermann, Gabriel A. Bonaterra, Ralf Kinscherf, Anja Schwarz
Atherosclerosis.2019; 281: 128. CrossRef
- The relationship of Growth differentiation factor-15 with renal damage and dyslipidemia in non-albuminuric and albuminuric Type-2 Diabetes Mellitus

 KDA
KDA
 First
First Prev
Prev





