
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
- Glucolipotoxicity Suppressed Autophagy and Insulin Contents in Human Islets, and Attenuation of PERK Activity Enhanced Them in an ATG7-Dependent Manner
- Seoil Moon, Ji Yoon Lim, Mirang Lee, Youngmin Han, Hongbeom Kim, Wooil Kwon, Jin-Young Jang, Mi Na Kim, Kyong Soo Park, Hye Seung Jung
- Diabetes Metab J. 2024;48(2):231-241. Published online September 6, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0366

- 1,705 View
- 157 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Administration of pancreatic endoplasmic reticulum kinase inhibitor (PERKi) improved insulin secretion and hyperglycemia in obese diabetic mice. In this study, autophagic balance was studied whether to mediate it.
Methods
Human islets were isolated from living patients without diabetes. PERKi GSK2606414 effects were evaluated in the islets under glucolipotoxicity by palmitate. Islet insulin contents and secretion were measured. Autophagic flux was assessed by microtubule associated protein 1 light chain 3 (LC3) conversion, a red fluorescent protein (RFP)-green fluorescent protein (GFP)- LC3 tandem assay, and P62 levels. For mechanical analyses, autophagy was suppressed using 3-methyladenine in mouse islets. Small interfering RNA for an autophagy-related gene autophagy related 7 (Atg7) was transfected to interfere autophagy.
Results
PERKi administration to mice decreased diabetes-induced P62 levels in the islets. Glucolipotoxicity significantly increased PERK phosphorylation by 70% and decreased insulin contents by 50% in human islets, and addition of PERKi (40 to 80 nM) recovered both. PERKi also enhanced glucose-stimulated insulin secretion (6-fold). PERKi up-regulated LC3 conversion suppressed by glucolipotoxicity, and down-regulated P62 contents without changes in P62 transcription, indicating enhanced autophagic flux. Increased autophagosome-lysosome fusion by PERKi was visualized in mouse islets, where PERKi enhanced ATG7 bound to LC3. Suppression of Atg7 eliminated PERKi-induced insulin contents and secretion.
Conclusion
This study provided functional changes of human islets with regard to autophagy under glucolipotoxicity, and suggested modulation of autophagy as an anti-diabetic mechanism of PERKi.
- Pathophysiology
- Endoplasmic Reticulum Stress and Dysregulated Autophagy in Human Pancreatic Beta Cells
- Seoil Moon, Hye Seung Jung
- Diabetes Metab J. 2022;46(4):533-542. Published online July 27, 2022
- DOI: https://doi.org/10.4093/dmj.2022.0070
- 4,540 View
- 250 Download
- 8 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
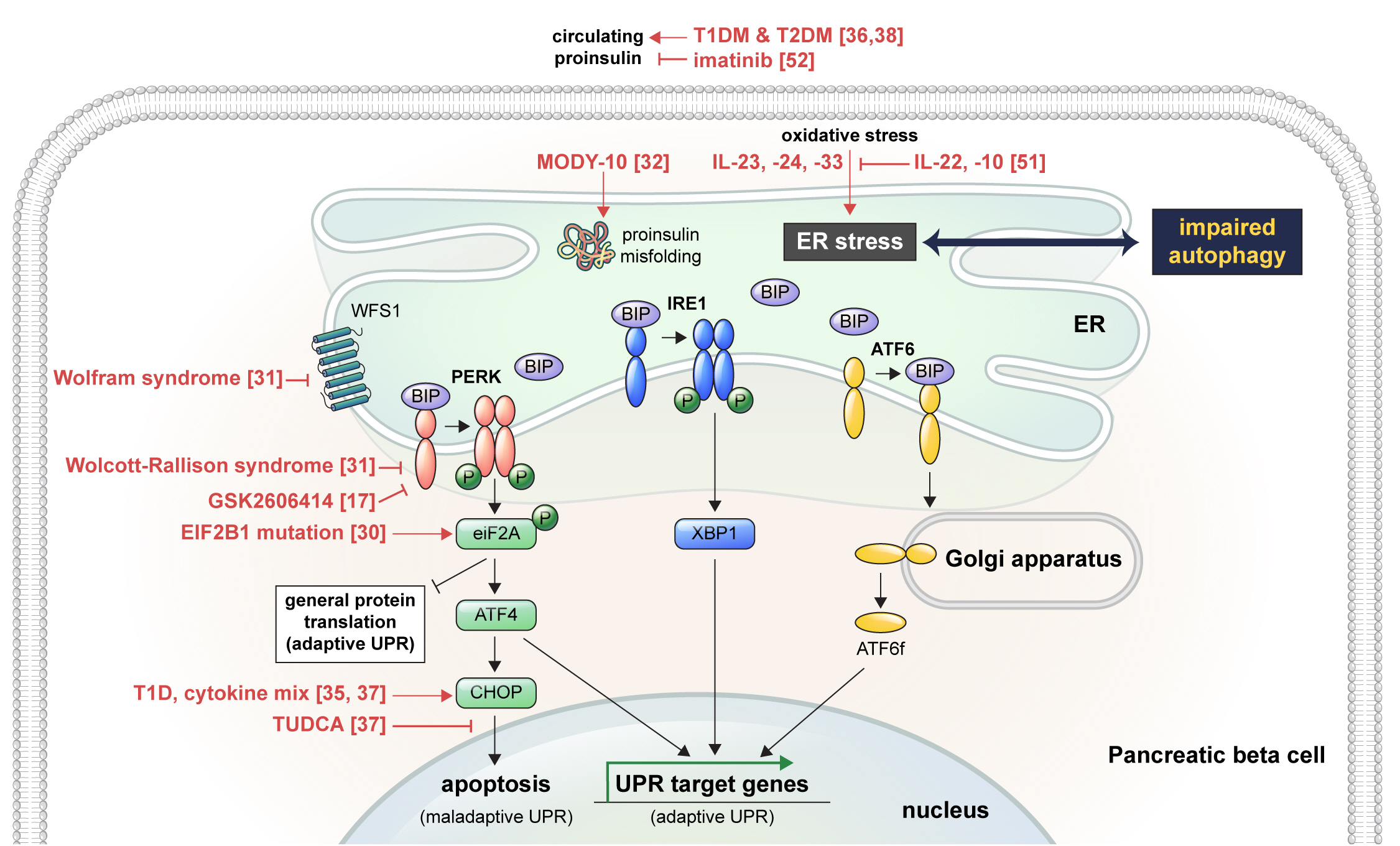
ePub - Pancreatic beta cell homeostasis is crucial for the synthesis and secretion of insulin; disruption of homeostasis causes diabetes, and is a treatment target. Adaptation to endoplasmic reticulum (ER) stress through the unfolded protein response (UPR) and adequate regulation of autophagy, which are closely linked, play essential roles in this homeostasis. In diabetes, the UPR and autophagy are dysregulated, which leads to beta cell failure and death. Various studies have explored methods to preserve pancreatic beta cell function and mass by relieving ER stress and regulating autophagic activity. To promote clinical translation of these research results to potential therapeutics for diabetes, we summarize the current knowledge on ER stress and autophagy in human insulin-secreting cells.
-
Citations
Citations to this article as recorded by- Glucolipotoxicity Suppressed Autophagy and Insulin Contents in Human Islets, and Attenuation of PERK Activity Enhanced Them in an ATG7-Dependent Manner
Seoil Moon, Ji Yoon Lim, Mirang Lee, Youngmin Han, Hongbeom Kim, Wooil Kwon, Jin-Young Jang, Mi Na Kim, Kyong Soo Park, Hye Seung Jung
Diabetes & Metabolism Journal.2024; 48(2): 231. CrossRef - Endoplasmic reticulum stress: A possible connection between intestinal inflammation and neurodegenerative disorders
Giorgio Vivacqua, Romina Mancinelli, Stefano Leone, Rosa Vaccaro, Ludovica Garro, Simone Carotti, Ludovica Ceci, Paolo Onori, Luigi Pannarale, Antonio Franchitto, Eugenio Gaudio, Arianna Casini
Neurogastroenterology & Motility.2024;[Epub] CrossRef - Docosahexanoic Acid Attenuates Palmitate-Induced Apoptosis by Autophagy Upregulation via GPR120/mTOR Axis in Insulin-Secreting Cells
Seok-Woo Hong, Jinmi Lee, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
Endocrinology and Metabolism.2024; 39(2): 353. CrossRef - Pancreatic islet remodeling in cotadutide-treated obese mice
Renata Spezani, Thatiany Souza Marinho, Luiz E. Macedo Cardoso, Marcia Barbosa Aguila, Carlos Alberto Mandarim-de-Lacerda
Life Sciences.2023; 327: 121858. CrossRef - Modulation of Unfolded Protein Response Restores Survival and Function of β-Cells Exposed to the Endocrine Disruptor Bisphenol A
Laura Maria Daian, Gabriela Tanko, Andrei Mircea Vacaru, Luiza Ghila, Simona Chera, Ana-Maria Vacaru
International Journal of Molecular Sciences.2023; 24(3): 2023. CrossRef - Interplay of skeletal muscle and adipose tissue: sarcopenic obesity
Min Jeong Park, Kyung Mook Choi
Metabolism.2023; 144: 155577. CrossRef - Identification and analysis of type 2 diabetes-mellitus-associated autophagy-related genes
Kun Cui, Zhizheng Li
Frontiers in Endocrinology.2023;[Epub] CrossRef - Sestrin2 in diabetes and diabetic complications
Xiaodan Zhang, Zirui Luo, Jiahong Li, Yaxuan Lin, Yu Li, Wangen Li
Frontiers in Endocrinology.2023;[Epub] CrossRef - Crosstalk between autophagy and insulin resistance: evidence from different tissues
Asie Sadeghi, Maryam Niknam, Mohammad Amin Momeni-Moghaddam, Maryam Shabani, Hamid Aria, Alireza Bastin, Maryam Teimouri, Reza Meshkani, Hamed Akbari
European Journal of Medical Research.2023;[Epub] CrossRef - Beta cell lipotoxicity in the development of type 2 diabetes: the need for species-specific understanding
Patricia Thomas, Meurig T. Gallagher, Gabriela Da Silva Xavier
Frontiers in Endocrinology.2023;[Epub] CrossRef
- Glucolipotoxicity Suppressed Autophagy and Insulin Contents in Human Islets, and Attenuation of PERK Activity Enhanced Them in an ATG7-Dependent Manner
- Basic Research
- DA-1241, a Novel GPR119 Agonist, Improves Hyperglycaemia by Inhibiting Hepatic Gluconeogenesis and Enhancing Insulin Secretion in Diabetic Mice
- Youjin Kim, Si Woo Lee, Hyejin Wang, Ryeong-Hyeon Kim, Hyun Ki Park, Hangkyu Lee, Eun Seok Kang
- Diabetes Metab J. 2022;46(2):337-348. Published online January 21, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0056
- 5,531 View
- 276 Download
- 8 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
We investigated the antidiabetic effects of DA-1241, a novel G protein-coupled receptor (GPR) 119 agonist, in vitro and in vivo.
Methods
DA-1241 was administrated to high-fat diet (HFD)-fed C57BL/6J mice for 12 weeks after hyperglycaemia developed. Oral/intraperitoneal glucose tolerance test and insulin tolerance test were performed. Serum insulin and glucagon-like peptide-1 (GLP-1) levels were measured during oral glucose tolerance test. Insulinoma cell line (INS-1E) cells and mouse islets were used to find whether DA-1241 directly stimulate insulin secretion in beta cell. HepG2 cells were used to evaluate the gluconeogenesis and autophagic process. Autophagic flux was evaluated by transfecting microtubule-associated protein 1 light chain 3-fused to green fluorescent protein and monomeric red fluorescent (mRFP-GFP-LC3) expression vector to HepG2 cells.
Results
Although DA-1241 treatment did not affect body weight gain and amount of food intake, fasting blood glucose level decreased along with increase in GLP-1 level. DA-1241 improved only oral glucose tolerance test and showed no effect in intraperitoneal glucose tolerance test. No significant effect was observed in insulin tolerance test. DA-1241 did not increase insulin secretion in INS-1E cell and mouse islets. DA-1241 reduced triglyceride content in the liver thereby improved fatty liver. Additionally, DA-1241 reduced gluconeogenic enzyme expression in HepG2 cells and mouse liver. DA-1241 reduced autophagic flow in HepG2 cells.
Conclusion
These findings suggested that DA-1241 augmented glucose-dependent insulin release via stimulation of GLP-1 secretion, and reduced hepatic gluconeogenesis, which might be associated with autophagic blockage, leading to improved glycaemic control. -
Citations
Citations to this article as recorded by- G protein-coupled receptors driven intestinal glucagon-like peptide-1 reprogramming for obesity: Hope or hype?
Mohan Patil, Ilaria Casari, Leon N. Warne, Marco Falasca
Biomedicine & Pharmacotherapy.2024; 172: 116245. CrossRef - GPR119 agonists for type 2 diabetes: past failures and future hopes for preclinical and early phase candidates
Deanne H Hryciw, Rhiannon K Patten, Raymond J Rodgers, Joseph Proietto, Dana S Hutchinson, Andrew J McAinch
Expert Opinion on Investigational Drugs.2024; 33(3): 183. CrossRef - Immunomodulation through Nutrition Should Be a Key Trend in Type 2 Diabetes Treatment
Katarzyna Napiórkowska-Baran, Paweł Treichel, Marta Czarnowska, Magdalena Drozd, Kinga Koperska, Agata Węglarz, Oskar Schmidt, Samira Darwish, Bartłomiej Szymczak, Zbigniew Bartuzi
International Journal of Molecular Sciences.2024; 25(7): 3769. CrossRef - Discovery of orally active sulfonylphenyl thieno[3,2-d]pyrimidine derivatives as GPR119 agonists
Heecheol Kim, Minjung Kim, Kyujin Oh, Sohee Lee, Sunyoung Lim, Sangdon Lee, Young Hoon Kim, Kwee Hyun Suh, Kyung Hoon Min
European Journal of Medicinal Chemistry.2023; 258: 115584. CrossRef - Increased expression of sodium-glucose cotransporter 2 and O-GlcNAcylation in hepatocytes drives non-alcoholic steatohepatitis
Hye Jin Chun, Eun Ran Kim, Minyoung Lee, Da Hyun Choi, Soo Hyun Kim, Eugene Shin, Jin-Hong Kim, Jin Won Cho, Dai Hoon Han, Bong-Soo Cha, Yong-ho Lee
Metabolism.2023; 145: 155612. CrossRef - Human skin stem cell-derived hepatic cells as in vitro drug discovery model for insulin-driven de novo lipogenesis
Karolien Buyl, Martine Vrints, Ruani Fernando, Terry Desmae, Thomas Van Eeckhoutte, Mia Jans, Jan Van Der Schueren, Joost Boeckmans, Robim M. Rodrigues, Veerle De Boe, Vera Rogiers, Joery De Kock, Filip Beirinckx, Tamara Vanhaecke
European Journal of Pharmacology.2023; 957: 175989. CrossRef - GPR119 activation by DA-1241 alleviates hepatic and systemic inflammation in MASH mice through inhibition of NFκB signaling
Seung-Ho Lee, Hansu Park, Eun-Kyoung Yang, Bo Ram Lee, Il-Hoon Jung, Tae-Hyoung Kim, Moon Jung Goo, Yuna Chae, Mi-Kyung Kim
Biomedicine & Pharmacotherapy.2023; 166: 115345. CrossRef - Characteristics of the Latest Therapeutic Agent for Diabetes
Nuri Yun
The Journal of Korean Diabetes.2023; 24(3): 148. CrossRef - DA-1241, a Novel GPR119 Agonist, Improves Hyperglycaemia by Inhibiting Hepatic Gluconeogenesis and Enhancing Insulin Secretion in Diabetic Mice
Youjin Kim, Si Woo Lee, Hyejin Wang, Ryeong-Hyeon Kim, Hyun Ki Park, Hangkyu Lee, Eun Seok Kang
Diabetes & Metabolism Journal.2022; 46(2): 337. CrossRef - Autophagy Dysregulation in Metabolic Associated Fatty Liver Disease: A New Therapeutic Target
Chun-Liang Chen, Yu-Cheng Lin
International Journal of Molecular Sciences.2022; 23(17): 10055. CrossRef
- G protein-coupled receptors driven intestinal glucagon-like peptide-1 reprogramming for obesity: Hope or hype?
- Basic Research
-
- The Effects of Exercise and Restriction of Sugar-Sweetened Beverages on Muscle Function and Autophagy Regulation in High-Fat High-Sucrose-Fed Obesity Mice
- Didi Zhang, Ji Hyun Lee, Hyung Eun Shin, Seong Eun Kwak, Jun Hyun Bae, Liang Tang, Wook Song
- Diabetes Metab J. 2021;45(5):773-786. Published online March 25, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0157
- 7,097 View
- 252 Download
- 5 Web of Science
- 6 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
- Background
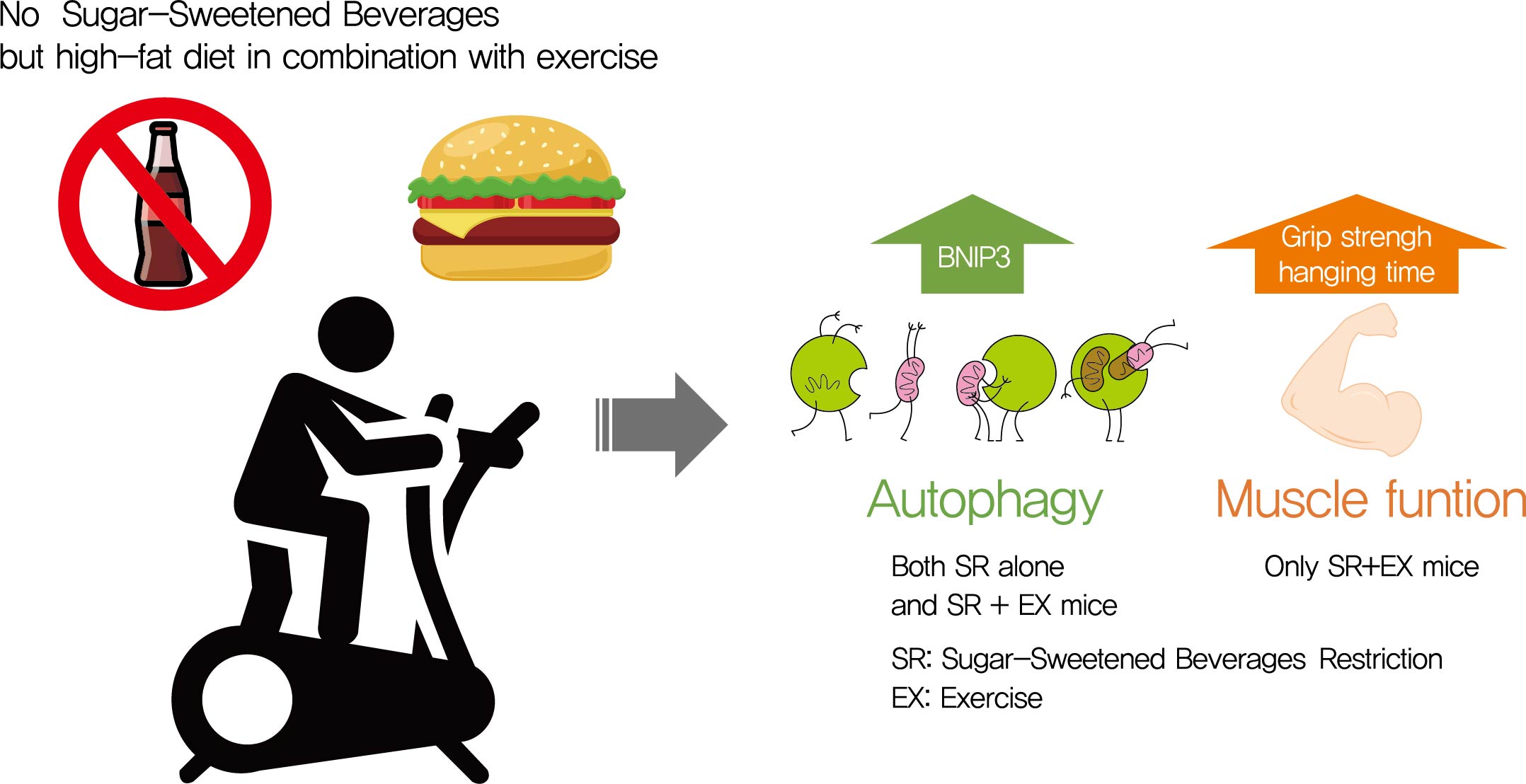
Autophagy maintains muscle mass and healthy skeletal muscles. Several recent studies have associated sugar-sweetened beverage (SSB) consumption with diseases. We investigated whether muscle dysfunction due to obesity could be restored by SSB restriction (SR) alone or in combination with exercise (EX) training.
Methods
Obese mice were subjected to SR combined with treadmill EX. Intraperitoneal glucose tolerance test, grip strength test, hanging time test, and body composition analysis were performed. Triglyceride (TG) and total cholesterol (TC) serum concentrations and TG concentrations in quadriceps muscles were analyzed. Western blot and reverse transcription-quantitative polymerase chain reaction helped analyze autophagy-related protein and mRNA expression, respectively.
Results
SR alone had no significant effect on fasting blood glucose levels, glucose tolerance, and muscle function. However, it had effect on serum TC, serum TG, and BCL2 interacting protein 3 expression. SR+EX improved glucose tolerance and muscle function and increased serum TC utilization than SR alone. SR+EX reduced P62 levels, increased glucose transporter type 4 and peroxisome proliferator-activated receptor γ coactivator-1α protein expression, and improved grip strength relative to the high-fat and high-sucrose liquid (HFHS) group, and this was not observed in the HFHS+EX group.
Conclusion
SR induced mitophagy-related protein expression in quadriceps, without affecting muscle function. And, the combination of SR and EX activated mitophagy-related proteins and improved muscle function. -
Citations
Citations to this article as recorded by- Mitochondrial Dysfunction, Oxidative Stress, and Inter-Organ Miscommunications in T2D Progression
Rajakrishnan Veluthakal, Diana Esparza, Joseph M. Hoolachan, Rekha Balakrishnan, Miwon Ahn, Eunjin Oh, Chathurani S. Jayasena, Debbie C. Thurmond
International Journal of Molecular Sciences.2024; 25(3): 1504. CrossRef - The association between healthy beverage index and sarcopenia in Iranian older adults: a case-control study
Marzieh Mahmoodi, Zainab Shateri, Mehran Nouri, Mohebat Vali, Nasrin Nasimi, Zahra Sohrabi, Mohammad Hossein Dabbaghmanesh, Maede Makhtoomi
BMC Geriatrics.2024;[Epub] CrossRef - Interplay of skeletal muscle and adipose tissue: sarcopenic obesity
Min Jeong Park, Kyung Mook Choi
Metabolism.2023; 144: 155577. CrossRef - Association between sugar-sweetened beverage consumption frequency and muscle strength: results from a sample of Chinese adolescents
Yunjie Zhang, Pan Xu, Yongjing Song, Nan Ma, Jinkui Lu
BMC Public Health.2023;[Epub] CrossRef - Muscle strength and prediabetes progression and regression in middle‐aged and older adults: a prospective cohort study
Shanhu Qiu, Xue Cai, Yang Yuan, Bo Xie, Zilin Sun, Duolao Wang, Tongzhi Wu
Journal of Cachexia, Sarcopenia and Muscle.2022; 13(2): 909. CrossRef - INTENSITY OF FREE RADICAL PROCESSES IN RAT SKELETAL MUSCLES UNDER THE CONDITIONS OF DIFFERENT DIETARY SUPPLY WITH NUTRIENTS
O.M. Voloshchuk, Н.P. Kopylchuk
Fiziolohichnyĭ zhurnal.2022; 68(4): 48. CrossRef
- Mitochondrial Dysfunction, Oxidative Stress, and Inter-Organ Miscommunications in T2D Progression
- Basic Research
- Role of Autophagy in Granulocyte-Colony Stimulating Factor Induced Anti-Apoptotic Effects in Diabetic Cardiomyopathy
- Guang-Yin Shen, Jeong-Hun Shin, Yi-Sun Song, Hyun-Woo Joo, In-Hwa Park, Jin-Hee Seong, Na-Kyoung Shin, A-Hyeon Lee, Young Jong Cho, Yonggu Lee, Young-Hyo Lim, Hyuck Kim, Kyung-Soo Kim
- Diabetes Metab J. 2021;45(4):594-605. Published online February 26, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0049
- 7,221 View
- 147 Download
- 3 Web of Science
- 2 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
- Background
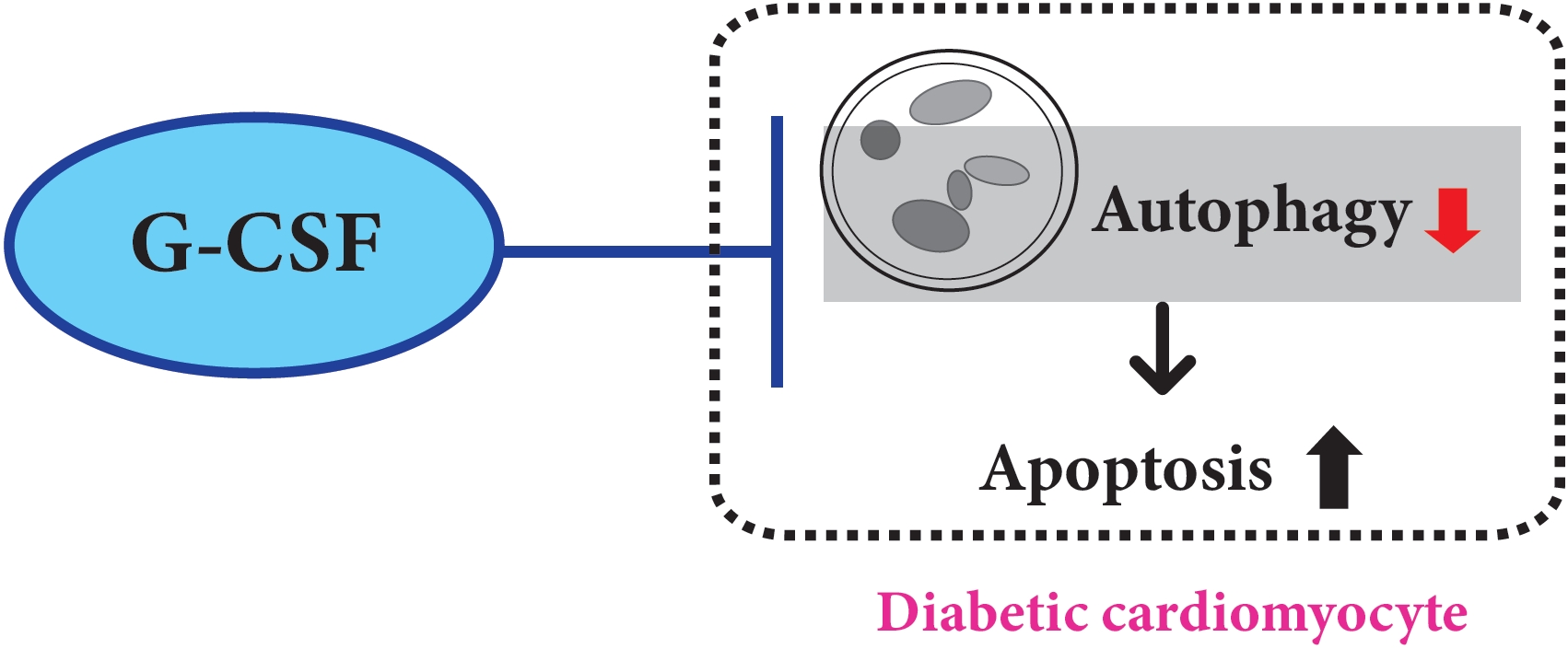
We previously, reported that granulocyte-colony stimulating factor (G-CSF) reduces cardiomyocyte apoptosis in diabetic cardiomyopathy. However, the underlying mechanisms are not yet fully understood. Therefore, we investigated whether the mechanisms underlying of the anti-apoptotic effects of G-CSF were associated with autophagy using a rat model of diabetic cardiomyopathy.
Methods
Diabetic cardiomyopathy was induced in rats through a high-fat diet combined with low-dose streptozotocin and the rats were then treated with G-CSF for 5 days. Rat H9c2 cardiac cells were cultured under high glucose conditions as an in vitro model of diabetic cardiomyopathy. The extent of apoptosis and protein levels related to autophagy (Beclin-1, microtubule-binding protein light chain 3 [LC3]-II/LC3-I ratio, and P62) were determined for both models. Autophagy determination was performed using an Autophagy Detection kit.
Results
G-CSF significantly reduced cardiomyocyte apoptosis in the diabetic myocardium in vivo and led to an increase in Beclin-1 level and the LC3-II/LC3-I ratio, and decreased P62 level. Similarly, G-CSF suppressed apoptosis, increased Beclin-1 level and LC3-II/LC3-I ratio, and decreased P62 level in high glucose-induced H9c2 cardiac cells in vitro. These effects of G-CSF were abrogated by 3-methyladenine, an autophagy inhibitor. In addition, G-CSF significantly increased autophagic flux in vitro.
Conclusion
Our results suggest that the anti-apoptotic effect of G-CSF might be significantly associated with the up-regulation of autophagy in diabetic cardiomyopathy. -
Citations
Citations to this article as recorded by- Ginkgo biloba extract protects against diabetic cardiomyopathy by restoring autophagy via adenosine monophosphate‐activated protein kinase/mammalian target of the rapamycin pathway modulation
Xueyao Yang, Xin Zhao, Yanfei Liu, Yue Liu, Libo Liu, Ziyu An, Haoran Xing, Jinfan Tian, Xiantao Song
Phytotherapy Research.2023; 37(4): 1377. CrossRef - Perspectives for Forkhead box transcription factors in diabetic cardiomyopathy: Their therapeutic potential and possible effects of salvianolic acids
Ronghui Han, Hemeng Huang, Weiyi Xia, Jingjin Liu, Hui Luo, Jing Tang, Zhengyuan Xia
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef
- Ginkgo biloba extract protects against diabetic cardiomyopathy by restoring autophagy via adenosine monophosphate‐activated protein kinase/mammalian target of the rapamycin pathway modulation
- Others
- Metformin Promotes Apoptosis but Suppresses Autophagy in Glucose-Deprived H4IIE Hepatocellular Carcinoma Cells
- Deok-Bae Park
- Diabetes Metab J. 2015;39(6):518-527. Published online December 11, 2015
- DOI: https://doi.org/10.4093/dmj.2015.39.6.518
- 4,119 View
- 42 Download
- 16 Web of Science
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Metformin, a well-known anti-diabetic drug, has gained interest due to its association with the reduction of the prevalence of cancer in patients with type 2 diabetes and the anti-proliferative effect of metformin in several cancer cells. Here, we investigated the anti-proliferative effect of metformin with respect to apoptosis and autophagy in H4IIE hepatocellular carcinoma cells.
Methods H4IIE rat cells were treated with metformin in glucose-free medium for 24 hours and were then subjected to experiments examining the onset of apoptosis and/or autophagy as well as the related signaling pathways.
Results When H4IIE cells were incubated in glucose-free media for 24 hours, metformin and 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) reduced the viability of cells. Inhibition of AMP-activated protein kinase (AMPK) by compound C significantly blocked cell death induced by metformin or AICAR. Pro-apoptotic events (nuclear condensation, hydrolysis of intact poly ADP ribose polymerase and caspase-3) were stimulated by metformin and then suppressed by compound C. Interestingly, the formation of acidic intracellular vesicles, a marker of autophagy, was stimulated by compound C. Although the deprivation of amino acids in culture media also induced apoptosis, neither metformin nor compound C affected cell viability. The expression levels of all of the autophagy-related proteins examined decreased with metformin, and two proteins (light chain 3 and beclin-1) were sensitive to compound C. Among the tested inhibitors against MAP kinases and phosphatidylinositol-3-kinase/mammalian target of rapamycin, SB202190 (against p38MAP kinase) significantly interrupted the effects of metformin.
Conclusion Our data suggest that metformin induces apoptosis, but suppresses autophagy, in hepatocellular carcinoma cells via signaling pathways, including AMPK and p38 mitogen-activated protein kinase.
-
Citations
Citations to this article as recorded by- Metformin Induces Lipogenesis and Apoptosis in H4IIE Hepatocellular
Carcinoma Cells
Deokbae Park, Sookyoung Lee, Hyejin Boo
Development & Reproduction.2023; 27(2): 77. CrossRef - Novel phloretin-based combinations targeting glucose metabolism in hepatocellular carcinoma through GLUT2/PEPCK axis of action: in silico molecular modelling and in vivo studies
Alaa Elmetwalli, Neamat H. Kamosh, Rania El Safty, Amany I. Youssef, Mohammed M. Salama, Khaled M. Abd El-Razek, Tarek El-Sewedy
Medical Oncology.2023;[Epub] CrossRef - Targeted Pyroptosis Is a Potential Therapeutic Strategy for Cancer
Hao Wu, Dianlun Qian, Xiangfeng Bai, Shibo Sun, Jayaprakash Narayana Kolla
Journal of Oncology.2022; 2022: 1. CrossRef - The effects of metformin on autophagy
Guangli Lu, Zhen Wu, Jia Shang, Zhenxing Xie, Chaoran Chen, Chuning zhang
Biomedicine & Pharmacotherapy.2021; 137: 111286. CrossRef - Protective Effect of Metformin against Hydrogen Peroxide-Induced Oxidative Damage in Human Retinal Pigment Epithelial (RPE) Cells by Enhancing Autophagy through Activation of AMPK Pathway
Xia Zhao, Linlin Liu, Yizhou Jiang, Marta Silva, Xuechu Zhen, Wenhua Zheng
Oxidative Medicine and Cellular Longevity.2020; 2020: 1. CrossRef Metformin Induces Autophagy via the AMPK-mTOR Signaling Pathway in Human Hepatocellular Carcinoma Cells
Chun Gao, Long Fang, Hui Zhang, Wei-Shuo Zhang, Xiao-Ou Li, Shi-Yu Du
Cancer Management and Research.2020; Volume 12: 5803. CrossRef- Metabolomics profiling of metformin-mediated metabolic reprogramming bypassing AMPKα
Min Yan, Huan Qi, Tian Xia, Xinjie Zhao, Wen Wang, Zhichao Wang, Chang Lu, Zhen Ning, Huan Chen, Tongming Li, Dinesh Singh Tekcham, Xiumei Liu, Jing Liu, Di Chen, Xiaolong Liu, Guowang Xu, Hai-long Piao
Metabolism.2019; 91: 18. CrossRef - Metformin Induces Oxidative Stress-Mediated Apoptosis without the Blockade of Glycolysis in H4IIE Hepatocellular Carcinoma Cells
Deokbae Park
Biological and Pharmaceutical Bulletin.2019; 42(12): 2002. CrossRef - Activation of AMPK prevents monocrotaline-induced pulmonary arterial hypertension by suppression of NF-κB-mediated autophagy activation
Cui Zhai, Wenhua Shi, Wei Feng, Yanting Zhu, Jian Wang, Shaojun Li, Xin Yan, Qingting Wang, Qianqian Zhang, Limin Chai, Cong Li, Pengtao Liu, Manxiang Li
Life Sciences.2018; 208: 87. CrossRef - Metformin and epothilone A treatment up regulate pro-apoptotic PARP-1, Casp-3 and H2AX genes and decrease of AKT kinase level to control cell death of human hepatocellular carcinoma and ovary adenocarcinoma cells
Aneta Rogalska, Barbara Bukowska, Agnieszka Marczak
Toxicology in Vitro.2018; 47: 48. CrossRef - Quantitative assessment of cell fate decision between autophagy and apoptosis
Bing Liu, Zoltán N. Oltvai, Hülya Bayır, Gary A. Silverman, Stephen C. Pak, David H. Perlmutter, Ivet Bahar
Scientific Reports.2017;[Epub] CrossRef - Meta-analysis of studies using metformin as a reducer for liver cancer risk in diabetic patients
Shujuan Ma, Yixiang Zheng, Yanni Xiao, Pengcheng Zhou, Hongzhuan Tan
Medicine.2017; 96(19): e6888. CrossRef - ROS Production and ERK Activity Are Involved in the Effects of d-β-Hydroxybutyrate and Metformin in a Glucose Deficient Condition
Santosh Lamichhane, Tonking Bastola, Ramesh Pariyar, Eun-Sol Lee, Ho-Sub Lee, Dae Lee, Jungwon Seo
International Journal of Molecular Sciences.2017; 18(3): 674. CrossRef - Metformin represses glucose starvation induced autophagic response in microvascular endothelial cells and promotes cell death
Samson Mathews Samuel, Suparna Ghosh, Yasser Majeed, Gnanapragasam Arunachalam, Mohamed M. Emara, Hong Ding, Chris R. Triggle
Biochemical Pharmacology.2017; 132: 118. CrossRef - NHX-5, an Endosomal Na+/H+ Exchanger, Is Associated with Metformin Action
Jeongho Kim, Hye-Yeon Lee, Jheesoo Ahn, Moonjung Hyun, Inhwan Lee, Kyung-Jin Min, Young-Jai You
Journal of Biological Chemistry.2016; 291(35): 18591. CrossRef - Metformin in pancreatic cancer treatment: from clinical trials through basic research to biomarker quantification
Archana Bhaw-Luximon, Dhanjay Jhurry
Journal of Cancer Research and Clinical Oncology.2016; 142(10): 2159. CrossRef - Metformina: stary lek w nowej aplikacji
Anna Dmoszyńska, Monika Podhorecka, Krzysztof Giannopoulos
Acta Haematologica Polonica.2016; 47(2): 139. CrossRef
- Metformin Induces Lipogenesis and Apoptosis in H4IIE Hepatocellular
Carcinoma Cells
- Complications
- Autophagy: A Novel Therapeutic Target for Diabetic Nephropathy
- Shinji Kume, Daisuke Koya
- Diabetes Metab J. 2015;39(6):451-460. Published online December 11, 2015
- DOI: https://doi.org/10.4093/dmj.2015.39.6.451
- 4,788 View
- 54 Download
- 79 Web of Science
- 73 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Diabetic nephropathy is a leading cause of end stage renal disease and its occurance is increasing worldwide. The most effective treatment strategy for the condition is intensive treatment to strictly control glycemia and blood pressure using renin-angiotensin system inhibitors. However, a fraction of patients still go on to reach end stage renal disease even under such intensive care. New therapeutic targets for diabetic nephropathy are, therefore, urgently needed. Autophagy is a major catabolic pathway by which mammalian cells degrade macromolecules and organelles to maintain intracellular homeostasis. The accumulation of damaged proteins and organelles is associated with the pathogenesis of diabetic nephropathy. Autophagy in the kidney is activated under some stress conditions, such as oxidative stress and hypoxia in proximal tubular cells, and occurs even under normal conditions in podocytes. These and other accumulating findings have led to a hypothesis that autophagy is involved in the pathogenesis of diabetic nephropathy. Here, we review recent findings underpinning this hypothesis and discuss the advantages of targeting autophagy for the treatment of diabetic nephropathy.
-
Citations
Citations to this article as recorded by- Aging and Diabetic Kidney Disease: Emerging Pathogenetic
Mechanisms and Clinical Implications
Yi Chen, Yashpal S. Kanwar, Xueqin Chen, Ming Zhan
Current Medicinal Chemistry.2024; 31(6): 697. CrossRef - Metformin inhibits high glucose‐induced apoptosis of renal podocyte through regulating miR‐34a/SIRT1 axis
Xudong Zhuang, Zhuye Sun, Huasheng Du, Tianhui Zhou, Jing Zou, Wei Fu
Immunity, Inflammation and Disease.2024;[Epub] CrossRef - Placenta-derived mesenchymal stem cells protect against diabetic kidney disease by upregulating autophagy-mediated SIRT1/FOXO1 pathway
Honghong Liu, Jiao Wang, Guanru Yue, Jixiong Xu
Renal Failure.2024;[Epub] CrossRef - Epigenetic Regulation of Autophagy in Bone Metabolism
Yazhou Zhang, Qianqian Wang, Hongjia Xue, Yujin Guo, Shanshan Wei, Fengfeng Li, Linqiang Gong, Weiliang Pan, Pei Jiang
Function.2024;[Epub] CrossRef - The interaction between lncRNAs and transcription factors regulating autophagy in human cancers: A comprehensive and therapeutical survey
Saade Abdalkareem Jasim, Yasir Qasim Almajidi, Reyadh R. Al‐Rashidi, Ahmed Hjazi, Irfan Ahmad, Ahmed Hussien Radie Alawadi, Enas R. Alwaily, Hashem O. Alsaab, Ali Haslany, Mohamood Hameed
Cell Biochemistry and Function.2024;[Epub] CrossRef - The beneficial effects of astragaloside IV on ameliorating diabetic kidney disease
Yiwei Gao, Xin Su, Taiqi Xue, Ning Zhang
Biomedicine & Pharmacotherapy.2023; 163: 114598. CrossRef - #2601 THE MOLECULAR EFFECT OF SGLT2I ON THE AUTOPHAGY PATHWAY IN TYPE II DIABETES MELLITUS AND ITS VASCULAR COMPLICATIONS
Offir Ertracht, Raneen Saad, Hagar Tadmor, Farid Nakhoul, Nakhoul Nakhoul
Nephrology Dialysis Transplantation.2023;[Epub] CrossRef - Role of oxidative stress in diabetes-induced complications and their management with antioxidants
Hasandeep Singh, Rajanpreet Singh, Arshdeep Singh, Harshbir Singh, Gurpreet Singh, Sarabjit Kaur, Balbir Singh
Archives of Physiology and Biochemistry.2023; : 1. CrossRef - Stress can affect mitochondrial energy metabolism and AMPK/SIRT1 signaling pathway in rats
An-ran Zhao, Jie Li, Si-qi Wang, Li-hua Bian, Wen-jing Li, Jian-you Guo
Brain Research Bulletin.2023; 203: 110770. CrossRef - Emerging links between FOXOs and diabetic complications
Urvi M. Parmar, Manjiri P. Jalgaonkar, Aayush J. Kansara, Manisha J. Oza
European Journal of Pharmacology.2023; 960: 176089. CrossRef - Pathophysiology of diabetic kidney disease and autophagy: A review
Jiawei Yu, Yan Liu, Hongjie Li, Peirong Zhang
Medicine.2023; 102(30): e33965. CrossRef - Microcystin-LR-induced autophagy via miR-282–5p/PIK3R1 pathway in Eriocheir sinensis hepatopancreas
Yuning Zhang, Jiancao Gao, Liping Cao, Jinliang Du, Gangchun Xu, Pao Xu
Ecotoxicology and Environmental Safety.2023; 267: 115661. CrossRef - Global research trends and hot spots on autophagy and kidney diseases: a bibliometric analysis from 2000 to 2022
Sinan Ai, Yake Li, Huijuan Zheng, Zhen Wang, Weijing Liu, JiaYin Tao, Yaotan Li, Yaoxian Wang
Frontiers in Pharmacology.2023;[Epub] CrossRef - Administration of mesenchymal stem cells in diabetic kidney disease: mechanisms, signaling pathways, and preclinical evidence
Yuexin Zhu, Manyu Luo, Xue Bai, Yan Lou, Ping Nie, Shan Jiang, Jicui Li, Bing Li, Ping Luo
Molecular and Cellular Biochemistry.2022; 477(8): 2073. CrossRef - Dictyophora Polysaccharide Attenuates As-Mediated PINK1/Parkin Pathway-Induced Mitophagy in L-02 Cell through Scavenging ROS
Ting Hu, Ju Lu, Changyan Wu, Tianxiao Duan, Peng Luo
Molecules.2022; 27(9): 2806. CrossRef - Asiatic acid from Cyclocarya paliurus regulates the autophagy–lysosome system via directly inhibiting TGF-β type I receptor and ameliorates diabetic nephropathy fibrosis
Xuan-xuan Zhang, Yao Liu, Su-su Xu, Ru Yang, Cui-hua Jiang, Li-ping Zhu, Yin-ying Xu, Ke Pan, Jian Zhang, Zhi-qi Yin
Food & Function.2022; 13(10): 5536. CrossRef - Therapeutic Potential of Resveratrol in Diabetic Nephropathy According
to Molecular Signaling
Marziyeh Salami, Raziyeh Salami, Alireza Mafi, Mohammad-Hossein Aarabi, Omid Vakili, Zatollah Asemi
Current Molecular Pharmacology.2022; 15(5): 716. CrossRef - Impact of SGLT2 inhibitors on the kidney in people with type 2 diabetes and severely increased albuminuria
Nasir Shah, Vlado Perkovic, Sradha Kotwal
Expert Review of Clinical Pharmacology.2022; 15(7): 827. CrossRef - Autophagy-nutrient sensing pathways in diabetic complications
Urvi M. Parmar, Manjiri P. Jalgaonkar, Yogesh A. Kulkarni, Manisha J. Oza
Pharmacological Research.2022; 184: 106408. CrossRef - The Molecular Effects of SGLT2i Empagliflozin on the Autophagy Pathway in Diabetes Mellitus Type 2 and Its Complications
Ranin Saad, Hagar Tadmor, Offir Ertracht, Nakhoul Nakhoul, Farid Nakhoul, Farber Evgeny, Shaul Atar, Bernd Stratmann
Journal of Diabetes Research.2022; 2022: 1. CrossRef - What’s New in the Molecular Mechanisms of Diabetic Kidney Disease: Recent Advances
Kimio Watanabe, Emiko Sato, Eikan Mishima, Mariko Miyazaki, Tetsuhiro Tanaka
International Journal of Molecular Sciences.2022; 24(1): 570. CrossRef - Autophagy blockade mechanistically links proton pump inhibitors to worsened diabetic nephropathy and aborts the renoprotection of metformin/enalapril
Dalia Kamal Mostafa, Mohamed Mostafa Khedr, Mervat Kamel Barakat, Amany Abdelbary Abdellatif, Amal Mohamed Elsharkawy
Life Sciences.2021; 265: 118818. CrossRef - Epigenetic modulation of autophagy genes linked to diabetic nephropathy by administration of isorhamnetin in Type 2 diabetes mellitus rats
Marwa Matboli, Doaa Ibrahim, Amany H Hasanin, Mohamed K Hassan, Eman K Habib, Miram M Bekhet, Ahmed M Afifi, Sanaa Eissa
Epigenomics.2021; 13(3): 187. CrossRef - Yishen capsule promotes podocyte autophagy through regulating SIRT1/NF-κB signaling pathway to improve diabetic nephropathy
Yuxiang Liu, Wenyuan Liu, Ziyuan Zhang, Yaling Hu, Xiaodong Zhang, Yanyan Sun, Qingqing Lei, Dalin Sun, Ting Liu, Yanjun Fan, Hui Li, Wujie Ding, Jingai Fang
Renal Failure.2021; 43(1): 128. CrossRef - Geniposide Improves Diabetic Nephropathy by Enhancing ULK1-Mediated Autophagy and Reducing Oxidative Stress through AMPK Activation
Theodomir Dusabimana, Eun Jung Park, Jihyun Je, Kyuho Jeong, Seung Pil Yun, Hye Jung Kim, Hwajin Kim, Sang Won Park
International Journal of Molecular Sciences.2021; 22(4): 1651. CrossRef - Update on diagnosis, pathophysiology, and management of diabetic kidney disease
Mai Sugahara, Wai Lun Will Pak, Tetsuhiro Tanaka, Sydney C. W. Tang, Masaomi Nangaku
Nephrology.2021; 26(6): 491. CrossRef - Life-Long Hyperbilirubinemia Exposure and Bilirubin Priming Prevent In Vitro Metabolic Damage
Annalisa Bianco, Serena Pinci, Claudio Tiribelli, Cristina Bellarosa
Frontiers in Pharmacology.2021;[Epub] CrossRef - NADH/NAD+ Redox Imbalance and Diabetic Kidney Disease
Liang-Jun Yan
Biomolecules.2021; 11(5): 730. CrossRef - Circular RNAs act as regulators of autophagy in cancer
Zhixia Zhou, Yinfeng Zhang, Jinning Gao, Xiaodan Hao, Chan Shan, Jing Li, Cuiyun Liu, Yin Wang, Peifeng Li
Molecular Therapy - Oncolytics.2021; 21: 242. CrossRef - Sarsasapogenin restores podocyte autophagy in diabetic nephropathy by targeting GSK3β signaling pathway
Xi-zhi Li, Hong Jiang, Liu Xu, Yi-qi Liu, Jia-wei Tang, Jia-sen Shi, Xiu-juan Yu, Xue Wang, Lei Du, Qian Lu, Cheng-lin Li, Yao-wu Liu, Xiao-xing Yin
Biochemical Pharmacology.2021; 192: 114675. CrossRef - Dietary Restriction for Kidney Protection: Decline in Nephroprotective Mechanisms During Aging
Nadezda V. Andrianova, Marina I. Buyan, Anastasia K. Bolikhova, Dmitry B. Zorov, Egor Y. Plotnikov
Frontiers in Physiology.2021;[Epub] CrossRef - Induction of PDCD4 by albumin in proximal tubule epithelial cells potentiates proteinuria-induced dysfunctional autophagy by negatively targeting Atg5
Ezra Kombo Osoro, Xiaojuan Du, Dong Liang, Xi Lan, Riaz Farooq, Fumeng Huang, Wenhua Zhu, Jiajun Ren, Muhammad Sadiq, Lifang Tian, Xudong Yang, Dongmin Li, Shemin Lu
Biochemistry and Cell Biology.2021; 99(5): 617. CrossRef - Overexpressing STAMP2 attenuates diabetic renal injuries via upregulating autophagy in diabetic rats
Fang-qiang Song, Ming Song, Wei-xuan Ma, Zhan Gao, Yun Ti, Xu Zhang, Bo-ang Hu, Ming Zhong, Wei Zhang, Ying Yu
Biochemical and Biophysical Research Communications.2021; 579: 47. CrossRef - Mitochondrial Regulation of Diabetic Kidney Disease
Daniel L. Galvan, Koki Mise, Farhad R. Danesh
Frontiers in Medicine.2021;[Epub] CrossRef - Diabetic kidney disease update: Pathogenesis and treatment overview for clinicians
Elmukhtar Habas, Abdel-Naser Elzouki
Journal of Diabetes and Endocrine Practice.2021; 04(03): 107. CrossRef - VDR/Atg3 Axis Regulates Slit Diaphragm to Tight Junction Transition via p62-Mediated Autophagy Pathway in Diabetic Nephropathy
Bin Wang, Jing-yi Qian, Tao-tao Tang, Li-lu Lin, Nan Yu, Hong-lei Guo, Wei-jie Ni, Ling-Li Lv, Yi Wen, Zuo-Lin Li, Min Wu, Jing-Yuan Cao, Bi-Cheng Liu
Diabetes.2021; 70(11): 2639. CrossRef - SIRT1: Mechanism and Protective Effect in Diabetic Nephropathy
Jing Ji, Pengyu Tao, Qian Wang, Lingxing Li, Yuzhen Xu
Endocrine, Metabolic & Immune Disorders - Drug Targets.2021; 21(5): 835. CrossRef - SIRT1 Alleviates Aldosterone-Induced Podocyte Injury by Suppressing Mitochondrial Dysfunction and NLRP3 Inflammasome Activation
Mingzhu Jiang, Min Zhao, Mi Bai, Juan Lei, Yanggang Yuan, Songming Huang, Yue Zhang, Guixia Ding, Zhanjun Jia, Aihua Zhang
Kidney Diseases.2021; 7(4): 293. CrossRef Salvianolic Acid B Improves Chronic Mild Stress-Induced Depressive Behaviors in Rats: Involvement of AMPK/SIRT1 Signaling Pathway
Dehua Liao, Yun Chen, Yujin Guo, Changshui Wang, Ni Liu, Qian Gong, Yingzhou Fu, Yilan Fu, Lizhi Cao, Dunwu Yao, Pei Jiang
Journal of Inflammation Research.2020; Volume 13: 195. CrossRef- Long non-coding RNAs and pyroptosis
Dong He, Jun Zheng, Jia Hu, Juan Chen, Xing Wei
Clinica Chimica Acta.2020; 504: 201. CrossRef - Resveratrol ameliorates renal damage by inhibiting oxidative stress-mediated apoptosis of podocytes in diabetic nephropathy
Fang Wang, Ran Li, Linlin Zhao, Shuang Ma, Guijun Qin
European Journal of Pharmacology.2020; 885: 173387. CrossRef - Beneficial Effect of Chloroquine and Amodiaquine on Type 1 Diabetic Tubulopathy by Attenuating Mitochondrial Nox4 and Endoplasmic Reticulum Stress
Jun Mo Kang, Hyun-Seob Lee, Junghyun Kim, Dong Ho Yang, Hye Yun Jeong, Yu Ho Lee, Dong-Jin Kim, Seon Hwa Park, MinJi Sung, Jaehee Kim, Hyun-Ju An, Sang Ho Lee, So-Young Lee
Journal of Korean Medical Science.2020;[Epub] CrossRef - Glucagon-like peptide-1 alleviates diabetic kidney disease through activation of autophagy by regulating AMP-activated protein kinase-mammalian target of rapamycin pathway
Shuangli Yang, Chuman Lin, Xiaoyun Zhuo, Jiyu Wang, Shitao Rao, Wen Xu, Yanzhen Cheng, Li Yang
American Journal of Physiology-Endocrinology and Metabolism.2020; 319(6): E1019. CrossRef - Inhibition of soluble epoxide hydrolase attenuates renal tubular mitochondrial dysfunction and ER stress by restoring autophagic flux in diabetic nephropathy
Xu-shun Jiang, Xing-yang Xiang, Xue-mei Chen, Jun-ling He, Ting Liu, Hua Gan, Xiao-gang Du
Cell Death & Disease.2020;[Epub] CrossRef - Orientin Protects Podocytes from High Glucose Induced Apoptosis through Mitophagy
Zi‐Li Kong, Kui Che, Jian‐Xia Hu, Ying Chen, Yun‐Yang Wang, Xiang Wang, Wen‐Shan Lü, Yan‐Gang Wang, Jing‐Wei Chi
Chemistry & Biodiversity.2020;[Epub] CrossRef - Sex-Specific Metabolic Changes in Peripheral Organs of Diabetic Mice
Xi Zhang, Hangying Xu, Jie Ning, Hui Ji, Junjie Yan, Yafei Zheng, Qingqing Xu, Chen Li, Liangcai Zhao, Hong Zheng, Hongchang Gao
Journal of Proteome Research.2020; 19(8): 3011. CrossRef - Liver X receptor activation induces podocyte injury via inhibiting autophagic activity
Ziyi Zhang, Shengjie Tang, Weiwei Gui, Xihua Lin, Fenping Zheng, Fang Wu, Hong Li
Journal of Physiology and Biochemistry.2020; 76(2): 317. CrossRef - Autophagy plays a protective role duringPseudomonas aeruginosa-induced apoptosis via ROS–MAPK pathway
Lu Han, Qinmei Ma, Jialin Yu, Zhaoqian Gong, Chenjie Ma, Yanan Xu, Guangcun Deng, Xiaoling Wu
Innate Immunity.2020; 26(7): 580. CrossRef - Autophagy in diabetic nephropathy: a review
Elias A. T. Koch, Rola Nakhoul, Farid Nakhoul, Nakhoul Nakhoul
International Urology and Nephrology.2020; 52(9): 1705. CrossRef - P2Y2R contributes to the development of diabetic nephropathy by inhibiting autophagy response
Theodomir Dusabimana, So Ra Kim, Eun Jung Park, Jihyun Je, Kyuho Jeong, Seung Pil Yun, Hye Jung Kim, Hwajin Kim, Sang Won Park
Molecular Metabolism.2020; 42: 101089. CrossRef - Cardioprotective Effects of Taurisolo® in Cardiomyoblast H9c2 Cells under High-Glucose and Trimethylamine N-Oxide Treatment via De Novo Sphingolipid Synthesis
Stefania Lama, Vincenzo Monda, Maria Rosaria Rizzo, Marco Dacrema, Maria Maisto, Giuseppe Annunziata, Gian Carlo Tenore, Ettore Novellino, Paola Stiuso, Laura Sartiani
Oxidative Medicine and Cellular Longevity.2020; 2020: 1. CrossRef - Empagliflozin attenuates diabetic tubulopathy by improving mitochondrial fragmentation and autophagy
Yu Ho Lee, Sang Hoon Kim, Jun Mo Kang, Jin Hyung Heo, Dong-Jin Kim, Seon Hwa Park, MinJi Sung, Jaehee Kim, Jisu Oh, Dong Ho Yang, Sang Ho Lee, So-Young Lee
American Journal of Physiology-Renal Physiology.2019; 317(4): F767. CrossRef - Critical role of mitochondrial dysfunction and impaired mitophagy in diabetic nephropathy
Sugandh Saxena, Alpana Mathur, Poonam Kakkar
Journal of Cellular Physiology.2019; 234(11): 19223. CrossRef - High Dose Vitamin E Attenuates Diabetic Nephropathy via Alleviation of Autophagic Stress
Yuxue Zhao, Wenting Zhang, Qi Jia, Zhendong Feng, Jing Guo, Xueting Han, Yuning Liu, Hongcai Shang, Yaoxian Wang, Wei Jing Liu
Frontiers in Physiology.2019;[Epub] CrossRef - Caffeic Acid Modulates miR-636 Expression in Diabetic Nephropathy Rats
Ahmed M. Salem, Aya S. Ragheb, Marwa G. A. Hegazy, Marwa Matboli, Sanaa Eissa
Indian Journal of Clinical Biochemistry.2019; 34(3): 296. CrossRef - Mechanistic Understanding of the Engineered Nanomaterial-Induced Toxicity on Kidney
Haiyang Zhao, Luxin Li, Huilu Zhan, Yanhui Chu, Bingbing Sun
Journal of Nanomaterials.2019; 2019: 1. CrossRef - Diabetic nephropathy: An update on pathogenesis and drug development
Vikram Rao A/L B Vasanth Rao, Sean Hong Tan, Mayuren Candasamy, Subrat Kumar Bhattamisra
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2019; 13(1): 754. CrossRef - Luteolin attenuates high glucose-induced podocyte injury via suppressing NLRP3 inflammasome pathway
Qian Yu, Minda Zhang, Lifen Qian, Dan Wen, Guanzhong Wu
Life Sciences.2019; 225: 1. CrossRef - Catalpol Ameliorates Podocyte Injury by Stabilizing Cytoskeleton and Enhancing Autophagy in Diabetic Nephropathy
Yan Chen, Qingpu Liu, Zengfu Shan, Wangyang Mi, Yingying Zhao, Meng Li, Baiyan Wang, Xiaoke Zheng, Weisheng Feng
Frontiers in Pharmacology.2019;[Epub] CrossRef - Role of sirtuin-1 in diabetic nephropathy
Wanning Wang, Weixia Sun, Yanli Cheng, Zhonggao Xu, Lu Cai
Journal of Molecular Medicine.2019; 97(3): 291. CrossRef - Energy restriction in renal protection
Si-Yang Wang, Guang-Yan Cai, Xiang-Mei Chen
British Journal of Nutrition.2018; 120(10): 1149. CrossRef - The dysregulated autophagy signaling is partially responsible for defective podocyte development in wt1a mutant zebrafish
Xuemei Zhang, Qiaohong Lin, Fan Ren, Jin Zhang, Farman Ullah Dawar, Jie Mei
Aquaculture and Fisheries.2018; 3(3): 99. CrossRef - Supplementation of Abelmoschus manihot Ameliorates Diabetic Nephropathy and Hepatic Steatosis by Activating Autophagy in Mice
Hwajin Kim, Theodomir Dusabimana, So Kim, Jihyun Je, Kyuho Jeong, Min Kang, Kye Cho, Hye Kim, Sang Park
Nutrients.2018; 10(11): 1703. CrossRef - Acute Kidney Injury and Progression of Diabetic Kidney Disease
Samuel Mon-Wei Yu, Joseph V. Bonventre
Advances in Chronic Kidney Disease.2018; 25(2): 166. CrossRef - Cardioprotective effects of dietary rapamycin on adult female C57BLKS/J‐Leprdb mice
Peter C. Reifsnyder, Sergey Ryzhov, Kevin Flurkey, Rea P. Anunciado‐Koza, Ian Mills, David E. Harrison, Robert A. Koza
Annals of the New York Academy of Sciences.2018; 1418(1): 106. CrossRef - Viability of primary cultured podocytes is associated with extracellular high glucose-dependent autophagy downregulation
Irena Audzeyenka, Dorota Rogacka, Agnieszka Piwkowska, Stefan Angielski, Maciej Jankowski
Molecular and Cellular Biochemistry.2017; 430(1-2): 11. CrossRef - Autophagy Protects against Palmitic Acid-Induced Apoptosis in Podocytes in vitro
Xu-shun Jiang, Xue-mei Chen, Jiang-min Wan, Hai-bo Gui, Xiong-zhong Ruan, Xiao-gang Du
Scientific Reports.2017;[Epub] CrossRef - Apelin involved in progression of diabetic nephropathy by inhibiting autophagy in podocytes
Yu Liu, Jia Zhang, Yangjia Wang, Xiangjun Zeng
Cell Death & Disease.2017; 8(8): e3006. CrossRef - Autophagy and its link to type II diabetes mellitus
Jai-Sing Yang, Chi-Cheng Lu, Sheng-Chu Kuo, Yuan-Man Hsu, Shih-Chang Tsai, Shih-Yin Chen, Yng-Tay Chen, Ying-Ju Lin, Yu-Chuen Huang, Chao-Jung Chen, Wei-De Lin, Wen-Lin Liao, Wei-Yong Lin, Yu-Huei Liu, Jinn-Chyuan Sheu, Fuu-Jen Tsai
BioMedicine.2017; 7(2): 8. CrossRef - Resveratrol protects podocytes against apoptosis via stimulation of autophagy in a mouse model of diabetic nephropathy
Shan-Shan Huang, Da-Fa Ding, Sheng Chen, Cheng-Long Dong, Xiao-Long Ye, Yang-Gang Yuan, Ya-Min Feng, Na You, Jia-Rong Xu, Heng Miao, Qiang You, Xiang Lu, Yi-Bing Lu
Scientific Reports.2017;[Epub] CrossRef - Long non-coding RNAs involved in autophagy regulation
Lixian Yang, Hanying Wang, Qi Shen, Lifeng Feng, Hongchuan Jin
Cell Death & Disease.2017; 8(10): e3073. CrossRef - Treatment of diabetic kidney disease: current and future targets
Mi-Kyung Kim
The Korean Journal of Internal Medicine.2017; 32(4): 622. CrossRef - MiR-30c protects diabetic nephropathy by suppressing epithelial-to-mesenchymal transition in db/db mice
Yanru Zhao, Zhongwei Yin, Huaping Li, Jiahui Fan, Shenglan Yang, Chen Chen, Dao Wen Wang
Aging Cell.2017; 16(2): 387. CrossRef
- Aging and Diabetic Kidney Disease: Emerging Pathogenetic
Mechanisms and Clinical Implications
- The Interplay between Autophagy and Aging
- Jong-Ok Pyo, Seung-Min Yoo, Yong-Keun Jung
- Diabetes Metab J. 2013;37(5):333-339. Published online October 17, 2013
- DOI: https://doi.org/10.4093/dmj.2013.37.5.333
- 3,791 View
- 37 Download
- 25 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Numerous studies have established a link between autophagy and aging; however, the relationship has not been clearly defined. Aging is a very complex process caused by the accumulation of various factors due to the gradual failure of cellular maintenance. Recent studies have shown that autophagy reduces the stress responses induced by starvation, reactive oxygen species, and the accumulation of intracellular proteins and organelles through cytoprotection, clearance of damaged mitochondria, and lysosomal degradation. Here, we summarize our current understanding of the relationship between autophagy and the aging process.
-
Citations
Citations to this article as recorded by- Dysregulation of autophagy activation induced by atorvastatin contributes to new-onset diabetes mellitus in western diet-fed mice
Juhee Kim, Minjune Kim, Minjeong Kim, Young-Hye You, Youngmi Song, Byung-Wan Lee
Metabolism.2024; 153: 155795. CrossRef - The impact of zinc on the molecular signaling pathways in the diabetes disease
Keyvan Asghari, Zahra Shargh, Sina Fatehfar, Leila Chodari, Parsa Sameei
Journal of Trace Elements in Medicine and Biology.2022; 72: 126985. CrossRef - Antiaging agents: safe interventions to slow aging and healthy life span extension
Ji-Kai Liu
Natural Products and Bioprospecting.2022;[Epub] CrossRef - Glycans in autophagy, endocytosis and lysosomal functions
Fulvio Reggiori, Hans-Joachim Gabius, Massimo Aureli, Winfried Römer, Sandro Sonnino, Eeva-Liisa Eskelinen
Glycoconjugate Journal.2021; 38(5): 625. CrossRef - Reduced cardiomyocyte Na+ current in the age‐dependent murine Pgc‐1β−/− model of ventricular arrhythmia
Shiraz Ahmad, Haseeb Valli, Robert Smyth, Anita Y. Jiang, Kamalan Jeevaratnam, Hugh R. Matthews, Christopher L.‐H. Huang
Journal of Cellular Physiology.2019; 234(4): 3921. CrossRef - Autophagy in Human Health and Disease: Novel Therapeutic Opportunities
Francesca Giampieri, Sadia Afrin, Tamara Y. Forbes-Hernandez, Massimiliano Gasparrini, Danila Cianciosi, Patricia Reboredo-Rodriguez, Alfonso Varela-Lopez, Jose L. Quiles, Maurizio Battino
Antioxidants & Redox Signaling.2019; 30(4): 577. CrossRef - Molybdenum Disulfide Nanoparticles Resist Oxidative Stress-Mediated Impairment of Autophagic Flux and Mitigate Endothelial Cell Senescence and Angiogenic Dysfunctions
Sunkui Ke, Youlin Lai, Tong Zhou, Lihuang Li, Yange Wang, Lei Ren, Shefang Ye
ACS Biomaterials Science & Engineering.2018; 4(2): 663. CrossRef - Phenformin inhibits cell proliferation and induces cell apoptosis and autophagy in cholangiocarcinoma
Shuyang Hu, Qing Ouyang, Qingbao Cheng, Jinghan Wang, Feiling Feng, Liang Qiao, Wei Gan, Yang Shi, Demin Wu, Xiaoqing Jiang
Molecular Medicine Reports.2018;[Epub] CrossRef - Ventricular pro-arrhythmic phenotype, arrhythmic substrate, ageing and mitochondrial dysfunction in peroxisome proliferator activated receptor-γ coactivator-1β deficient (Pgc-1β) murine hearts
Shiraz Ahmad, Haseeb Valli, Karan R. Chadda, James Cranley, Kamalan Jeevaratnam, Christopher L.-H. Huang
Mechanisms of Ageing and Development.2018; 173: 92. CrossRef - From Christian de Duve to Yoshinori Ohsumi: More to autophagy than just dining at home
Margaret M. Harnett, Miguel A. Pineda, Perle Latré de Laté, Russell J. Eason, Sébastien Besteiro, William Harnett, Gordon Langsley
Biomedical Journal.2017; 40(1): 9. CrossRef - Metformin Restores Parkin-Mediated Mitophagy, Suppressed by Cytosolic p53
Young Song, Woo Lee, Yong-ho Lee, Eun Kang, Bong-Soo Cha, Byung-Wan Lee
International Journal of Molecular Sciences.2016; 17(1): 122. CrossRef - Selected reaction monitoring mass spectrometry for relative quantification of proteins involved in cellular life and death processes
Rune Isak Dupont Birkler, Zahra Nochi, Niels Gregersen, Johan Palmfeldt
Journal of Chromatography B.2016; 1035: 49. CrossRef - Dehydroepiandrosterone prevents linoleic acid-induced endothelial cell senescence by increasing autophagy
Min Jung Lee, Eun Hee Kim, Sang Ah. Lee, Yu Mi Kang, Chang Hee Jung, Hae Kyeong Yoon, So Mi Seol, Yoo La Lee, Woo Je Lee, Joong-Yeol Park
Metabolism.2015; 64(9): 1134. CrossRef - Successful aging: Advancing the science of physical independence in older adults
Stephen D. Anton, Adam J. Woods, Tetso Ashizawa, Diana Barb, Thomas W. Buford, Christy S. Carter, David J. Clark, Ronald A. Cohen, Duane B. Corbett, Yenisel Cruz-Almeida, Vonetta Dotson, Natalie Ebner, Philip A. Efron, Roger B. Fillingim, Thomas C. Foster
Ageing Research Reviews.2015; 24: 304. CrossRef - Autophagy in acute leukemias: A double-edged sword with important therapeutic implications
Cecilia Evangelisti, Camilla Evangelisti, Francesca Chiarini, Annalisa Lonetti, Francesca Buontempo, Luca M. Neri, James A. McCubrey, Alberto M. Martelli
Biochimica et Biophysica Acta (BBA) - Molecular Cell Research.2015; 1853(1): 14. CrossRef - Autophagy: A housekeeper in cardiorenal metabolic health and disease
Guanghong Jia, James R. Sowers
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2015; 1852(2): 219. CrossRef - Autophagy and Lipid Droplets in the Liver
Nuria Martinez-Lopez, Rajat Singh
Annual Review of Nutrition.2015; 35(1): 215. CrossRef - Lysosomal Two-pore Channel Subtype 2 (TPC2) Regulates Skeletal Muscle Autophagic Signaling
Pei-Hui Lin, Pu Duann, Shinji Komazaki, Ki Ho Park, Haichang Li, Mingzhai Sun, Mathew Sermersheim, Kristyn Gumpper, John Parrington, Antony Galione, A. Mark Evans, Michael X. Zhu, Jianjie Ma
Journal of Biological Chemistry.2015; 290(6): 3377. CrossRef - Zinc and autophagy
Juan P. Liuzzi, Liang Guo, Changwon Yoo, Tiffanie S. Stewart
BioMetals.2014; 27(6): 1087. CrossRef - Autophagy in the eye: Implications for ocular cell health
Laura S. Frost, Claire H. Mitchell, Kathleen Boesze-Battaglia
Experimental Eye Research.2014; 124: 56. CrossRef - Can Enhanced Autophagy Be Associated with Human Longevity? Serum Levels of the Autophagy Biomarker Beclin-1 Are Increased in Healthy Centenarians
Enzo Emanuele, Piercarlo Minoretti, Fabian Sanchis-Gomar, Helios Pareja-Galeano, Yusuf Yilmaz, Nuria Garatachea, Alejandro Lucia
Rejuvenation Research.2014; 17(6): 518. CrossRef - Responses and adaptations of intervertebral disc cells to microenvironmental stress: a possible central role of autophagy in the adaptive mechanism
Libo Jiang, Fenglai Yuan, Xiaofan Yin, Jian Dong
Connective Tissue Research.2014; 55(5-6): 311. CrossRef - STOP accelerating lung aging for the treatment of COPD
Kazuhiro Ito, Nicolas Mercado
Experimental Gerontology.2014; 59: 21. CrossRef - In search of antiaging modalities: Evaluation of mTOR‐ and ROS/DNA damage‐signaling by cytometry
Zbigniew Darzynkiewicz, Hong Zhao, H. Dorota Halicka, Jiangwei Li, Yong‐Syu Lee, Tze‐Chen Hsieh, Joseph M. Wu
Cytometry Part A.2014; 85(5): 386. CrossRef - Induction of Covalently Crosslinked p62 Oligomers with Reduced Binding to Polyubiquitinated Proteins by the Autophagy Inhibitor Verteporfin
Elizabeth Donohue, Aruna D. Balgi, Masaaki Komatsu, Michel Roberge, Srinivasa M. Srinivasula
PLoS ONE.2014; 9(12): e114964. CrossRef
- Dysregulation of autophagy activation induced by atorvastatin contributes to new-onset diabetes mellitus in western diet-fed mice
- Nutritional Status and Cardiac Autophagy
- Jihyun Ahn, Jaetaek Kim
- Diabetes Metab J. 2013;37(1):30-35. Published online February 15, 2013
- DOI: https://doi.org/10.4093/dmj.2013.37.1.30
- 3,570 View
- 34 Download
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Autophagy is necessary for the degradation of long-lasting proteins and nonfunctional organelles, and is activated to promote cellular survival. However, overactivation of autophagy may deplete essential molecules and organelles responsible for cellular survival. Lifelong calorie restriction by 40% has been shown to increase the cardiac expression of autophagic markers, which suggests that it may have a cardioprotective effect by decreasing oxidative damage brought on by aging and cardiovascular diseases. Although cardiac autophagy is critical to regulating protein quality and maintaining cellular function and survival, increased or excessive autophagy may have deleterious effects on the heart under some circumstances, including pressure overload-induced heart failure. The importance of autophagy has been shown in nutrient supply and preservation of energy in times of limitation, such as ischemia. Some studies have suggested that a transition from obesity to metabolic syndrome may involve progressive changes in myocardial inflammation, mitochondrial dysfunction, fibrosis, apoptosis, and myocardial autophagy.
-
Citations
Citations to this article as recorded by- The Newly Proposed Mechanism of Cardiomyocyte Protection of Carvedilol-
Anti-Apoptosis Pattern of Carvedilol in Anoxia by Inducing Autophagy
Partly through the AMPK/mTOR Pathway
Jingru Li, Chaozhong Li, Guihu Sun, Longjun Li, Yongli Zeng, Huawei Wang, Xinyu Wu, Ping Yang, Yunzhu Peng, Luqiao Wang
Letters in Drug Design & Discovery.2023; 20(10): 1600. CrossRef - Mitophagy for cardioprotection
Allen Sam Titus, Eun-Ah Sung, Daniela Zablocki, Junichi Sadoshima
Basic Research in Cardiology.2023;[Epub] CrossRef - Main active components of Si-Miao-Yong-An decoction (SMYAD) attenuate autophagy and apoptosis via the PDE5A-AKT and TLR4-NOX4 pathways in isoproterenol (ISO)-induced heart failure models
Minru Liao, Qiang Xie, Yuqian Zhao, Chengcan Yang, Congcong Lin, Guan Wang, Bo Liu, Lingjuan Zhu
Pharmacological Research.2022; 176: 106077. CrossRef - How Can Malnutrition Affect Autophagy in Chronic Heart Failure? Focus and Perspectives
Giovanni Corsetti, Evasio Pasini, Claudia Romano, Carol Chen-Scarabelli, Tiziano M. Scarabelli, Vincenzo Flati, Louis Saravolatz, Francesco S. Dioguardi
International Journal of Molecular Sciences.2021; 22(7): 3332. CrossRef - Ischemia reperfusion injury induces pyroptosis and mediates injury in steatotic liver thorough Caspase 1 activation
Vasantha L. Kolachala, Chrissy Lopez, Ming Shen, Dmitry Shayakhmetov, Nitika Arora Gupta
Apoptosis.2021; 26(5-6): 361. CrossRef - Metformin prevents against oxidative stress-induced senescence in human periodontal ligament cells
Yunchun Kuang, Bo Hu, Ge Feng, Mingli Xiang, Yuejia Deng, Minmin Tan, Jie Li, Jinlin Song
Biogerontology.2020; 21(1): 13. CrossRef - Protective effects of salvianolic acid B against hydrogen peroxide‑induced apoptosis of human umbilical vein endothelial cells and underlying mechanisms
Shan Gao, Shiqin Li, Qin Li, Fuyong Zhang, Mengqi Sun, Zilin Wan, Shurong Wang
International Journal of Molecular Medicine.2019;[Epub] CrossRef - Moderate calorie restriction attenuates age‑associated alterations and improves cardiac function by increasing SIRT1 and SIRT3 expression
Wei Yu, Jinjin Qin, Chunjuan Chen, Yucai Fu, Wei Wang
Molecular Medicine Reports.2018;[Epub] CrossRef - Cardiac fibrosis in the ageing heart: Contributors and mechanisms
Lu Lu, Jingbin Guo, Yue Hua, Kevin Huang, Ruth Magaye, Jake Cornell, Darren J. Kelly, Christopher Reid, Danny Liew, Yingchun Zhou, Aihua Chen, Wei Xiao, Qiang Fu, Bing Hui Wang
Clinical and Experimental Pharmacology and Physiology.2017; 44(S1): 55. CrossRef - MicroRNA-199a acts as a potential suppressor of cardiomyocyte autophagy through targeting Hspa5
Liang Chen, Fei-Yu Wang, Zhen-Yu Zeng, Ling Cui, Jian Shen, Xiao-Wei Song, Pan Li, Xian-Xian Zhao, Yong-Wen Qin
Oncotarget.2017; 8(38): 63825. CrossRef - Protective effects of luteolin-7-O-glucoside against starvation-induced injury through upregulation of autophagy in H9c2 Cells
Hong Yao, Lichun Zhou, Linlin Tang, Yanhui Guan, Shang Chen, Yu Zhang, Xiuzhen Han
BioScience Trends.2017; 11(5): 557. CrossRef - Hongjingtian Injection Attenuates Myocardial Oxidative Damage via Promoting Autophagy and Inhibiting Apoptosis
Shujing Zhang, Ling Zhang, Han Zhang, Guanwei Fan, Jiuwen Qiu, Zongbao Fang, Haibo Wu, Yi Wang, Xiaoping Zhao
Oxidative Medicine and Cellular Longevity.2017; 2017: 1. CrossRef - Autophagy is a regulator of TGF-β1-induced fibrogenesis in primary human atrial myofibroblasts
S Ghavami, R H Cunnington, S Gupta, B Yeganeh, K L Filomeno, D H Freed, S Chen, T Klonisch, A J Halayko, E Ambrose, R Singal, I M C Dixon
Cell Death & Disease.2015; 6(3): e1696. CrossRef - The number of cardiac myocytes in the hypertrophic and hypotrophic left ventricle of the obese and calorie‐restricted mouse heart
Julia Schipke, Ewgenija Banmann, Sandeep Nikam, Robert Voswinckel, Karin Kohlstedt, Annemarieke E. Loot, Ingrid Fleming, Christian Mühlfeld
Journal of Anatomy.2014; 225(5): 539. CrossRef - Glycated Albumin Causes Pancreatic β-Cells Dysfunction Through Autophagy Dysfunction
Young Mi Song, Sun Ok Song, Young-Hye You, Kun-Ho Yoon, Eun Seok Kang, Bong Soo Cha, Hyun Chul Lee, Ji-Won Kim, Byung-Wan Lee
Endocrinology.2013; 154(8): 2626. CrossRef - Cardiac Metabolism and its Interactions With Contraction, Growth, and Survival of Cardiomyocytes
Stephen C. Kolwicz, Suneet Purohit, Rong Tian
Circulation Research.2013; 113(5): 603. CrossRef
- The Newly Proposed Mechanism of Cardiomyocyte Protection of Carvedilol-
Anti-Apoptosis Pattern of Carvedilol in Anoxia by Inducing Autophagy
Partly through the AMPK/mTOR Pathway
- Autophagy in Diabetes.
- Hye Seung Jung, Myung Shik Lee
- Korean Diabetes J. 2009;33(6):453-457. Published online December 1, 2009
- DOI: https://doi.org/10.4093/kdj.2009.33.6.453
- 1,843 View
- 25 Download
-
 Abstract
Abstract
 PDF
PDF - Diabetes mellitus is characterized by decreased insulin secretion and action. Decreased insulin secretion results from a reduction in mass and/or function of pancreatic beta-cells. Apoptosis, oxidative stress, mitochondrial dysfunction, and endoplasmic reticulum (ER) stress responses have been suggested as mechanisms for the changes in beta-cells in type 2 diabetes; however, the underlying causes have not been clearly elucidated. Autophagy is an intracellular process that maintains cellular homeostasis through degradation and recycling of organelles. Recently, we reported reduction of beta-cell mass in autophagy-deficient mice. Pancreatic insulin content was also decreased due to the decreased beta-cell mass and the reduced number of insulin granules. Morphological analysis of these beta-cells revealed an accumulation of ubiquitinated proteins, swollen mitochondria, and distended ER. Insulin secretory function ex vivo was also impaired. As a result, autophagy-deficient mice showed hypoinsulinemia and hyperglycemia. These results suggested that autophagy is necessary to maintain the structure, mass and function of beta-cells. In addition, as autophagy may play a protective role against ER stress and rejuvenate organelle function, impaired autophagy may lead to mitochondrial dysfunction and ER stress, which have been implicated as causes of insulin resistance. Therefore, in addition to beta-cell homeostasis, dysregulated autophagy may possibly be involved in insulin resistance.

 KDA
KDA
 First
First Prev
Prev





