
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition
- Ho Gyun Lee, Il Hyeon Jung, Byong Seo Park, Hye Rim Yang, Kwang Kon Kim, Thai Hien Tu, Jung-Yong Yeh, Sewon Lee, Sunggu Yang, Byung Ju Lee, Jae Geun Kim, Il Seong Nam-Goong
- Diabetes Metab J. 2023;47(6):784-795. Published online August 23, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0261
- 1,417 View
- 149 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
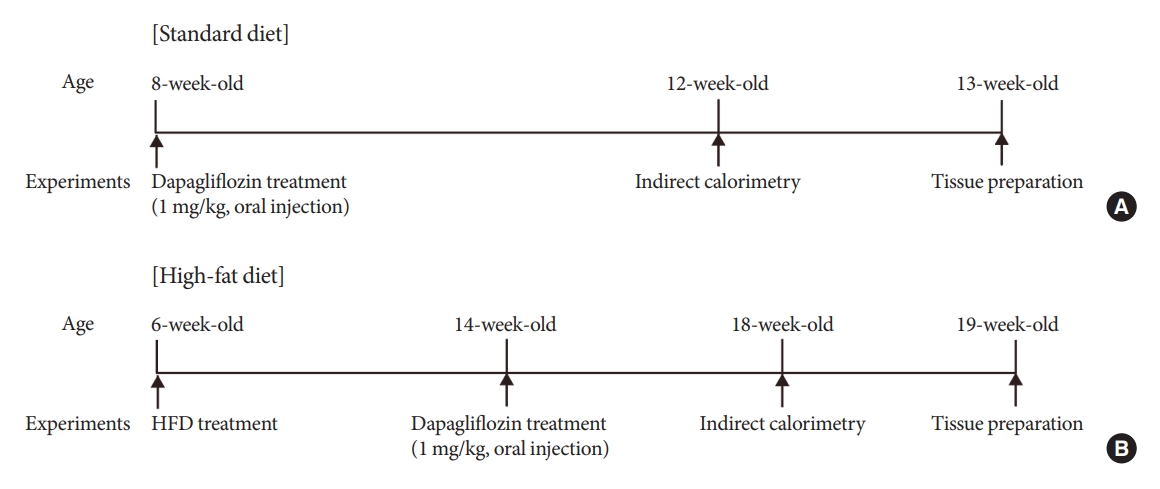
Sodium-glucose cotransporter 2 (SGLT-2) inhibitors are currently used to treat patients with diabetes. Previous studies have demonstrated that treatment with SGLT-2 inhibitors is accompanied by altered metabolic phenotypes. However, it has not been investigated whether the hypothalamic circuit participates in the development of the compensatory metabolic phenotypes triggered by the treatment with SGLT-2 inhibitors.
Methods
Mice were fed a standard diet or high-fat diet and treated with dapagliflozin, an SGLT-2 inhibitor. Food intake and energy expenditure were observed using indirect calorimetry system. The activity of hypothalamic neurons in response to dapagliflozin treatment was evaluated by immunohistochemistry with c-Fos antibody. Quantitative real-time polymerase chain reaction was performed to determine gene expression patterns in the hypothalamus of dapagliflozin-treated mice.
Results
Dapagliflozin-treated mice displayed enhanced food intake and reduced energy expenditure. Altered neuronal activities were observed in multiple hypothalamic nuclei in association with appetite regulation. Additionally, we found elevated immunosignals of agouti-related peptide neurons in the paraventricular nucleus of the hypothalamus.
Conclusion
This study suggests the functional involvement of the hypothalamus in the development of the compensatory metabolic phenotypes induced by SGLT-2 inhibitor treatment. -
Citations
Citations to this article as recorded by- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
Jae Hyun Bae
Diabetes & Metabolism Journal.2024; 48(1): 157. CrossRef - Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
Ho Gyun Lee, Il Hyeon Jung, Byong Seo Park, Hye Rim Yang, Kwang Kon Kim, Thai Hien Tu, Jung-Yong Yeh, Sewon Lee, Sunggu Yang, Byung Ju Lee, Jae Geun Kim, Il Seong Nam-Goong
Diabetes & Metabolism Journal.2024; 48(1): 159. CrossRef
- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
- Drug/Regimen
-
- Glucagon-Like Peptide-1 Receptor Agonist Differentially Affects Brain Activation in Response to Visual Food Cues in Lean and Obese Individuals with Type 2 Diabetes Mellitus
- Jae Hyun Bae, Hyung Jin Choi, Kang Ik Kevin Cho, Lee Kyung Kim, Jun Soo Kwon, Young Min Cho
- Diabetes Metab J. 2020;44(2):248-259. Published online November 4, 2019
- DOI: https://doi.org/10.4093/dmj.2019.0018
- 7,388 View
- 222 Download
- 5 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background To investigate the effects of a glucagon-like peptide-1 receptor agonist on functional brain activation in lean and obese individuals with type 2 diabetes mellitus (T2DM) in response to visual food cues.
Methods In a randomized, single-blinded, crossover study, 15 lean and 14 obese individuals with T2DM were administered lixisenatide or normal saline subcutaneously with a 1-week washout period. We evaluated brain activation in response to pictures of high-calorie food, low-calorie food, and nonfood using functional magnetic resonance imaging and measured appetite and caloric intake in participants who were given access to an
ad libitum buffet.Results Obese individuals with T2DM showed significantly greater activation of the hypothalamus, pineal gland, parietal cortex (high-calorie food vs. low-calorie food,
P <0.05), orbitofrontal cortex (high-calorie food vs. nonfood,P <0.05), and visual cortex (food vs. nonfood,P <0.05) than lean individuals with T2DM. Lixisenatide injection significantly reduced the functional activation of the fusiform gyrus and lateral ventricle in obese individuals with T2DM compared with that in lean individuals with T2DM (nonfood vs. high-calorie food,P <0.05). In addition, in individuals who decreased their caloric intake after lixisenatide injection, there were significant interaction effects between group and treatment in the posterior cingulate, medial frontal cortex (high-calorie food vs. low-calorie food,P <0.05), hypothalamus, orbitofrontal cortex, and temporal lobe (food vs. nonfood,P <0.05).Conclusion Brain responses to visual food cues were different in lean and obese individuals with T2DM. In addition, acute administration of lixisenatide differentially affected functional brain activation in these individuals, especially in those who decreased their caloric intake after lixisenatide injection.
-
Citations
Citations to this article as recorded by- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
Jae Hyun Bae
Diabetes & Metabolism Journal.2024; 48(1): 157. CrossRef - Diabetes remission and relapse following an intensive metabolic intervention combining insulin glargine/lixisenatide, metformin and lifestyle approaches: Results of a randomised controlled trial
Natalia McInnes, Stephanie Hall, Heather A. Lochnan, Stewart B. Harris, Zubin Punthakee, Ronald J. Sigal, Irene Hramiak, Mohammed Azharuddin, Joanne F. Liutkus, Jean‐François Yale, Farah Sultan, Ada Smith, Rose E. Otto, Diana Sherifali, Yan Yun Liu, Hertz
Diabetes, Obesity and Metabolism.2023; 25(11): 3347. CrossRef - Glucagon-like peptide-1 analog therapy in rare genetic diseases: monogenic obesity, monogenic diabetes, and spinal muscular atrophy
Hussein Zaitoon, Ronit Lubetzky, Achiya Z. Amir, Hadar Moran-Lev, Liora Sagi, Michal Yacobi-Bach, Ophir Borger, Efrat Chorna, Yael Lebenthal, Avivit Brener
Acta Diabetologica.2023; 60(8): 1099. CrossRef - What can functional brain imaging teach us about remission of type 2 diabetes?
Dhruti Hirani, Shahd Alabdulkader, Alexander. D. Miras, Victoria Salem
Diabetic Medicine.2023;[Epub] CrossRef - Fasting oxyntomodulin, glicentin, and gastric inhibitory polypeptide levels are associated with activation of reward‐ and attention‐related brain centres in response to visual food cues in adults with obesity: A cross‐sectional functional MRI study
Nikolaos Perakakis, Olivia M. Farr, Christos S. Mantzoros
Diabetes, Obesity and Metabolism.2021; 23(5): 1202. CrossRef - Aberrant Brain Functional Connectivity Strength and Effective Connectivity in Patients with Type 2 Diabetes Mellitus
Xi Guo, Su Wang, Yu-Chen Chen, Heng-Le Wei, Gang-Ping Zhou, Yu-Sheng Yu, Xindao Yin, Kun Wang, Hong Zhang, Eusebio Chiefari
Journal of Diabetes Research.2021; 2021: 1. CrossRef
- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
- Molecular Mechanisms of Appetite Regulation
- Ji Hee Yu, Min-Seon Kim
- Diabetes Metab J. 2012;36(6):391-398. Published online December 12, 2012
- DOI: https://doi.org/10.4093/dmj.2012.36.6.391
- 8,979 View
- 215 Download
- 80 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The prevalence of obesity has been rapidly increasing worldwide over the last several decades and has become a major health problem in developed countries. The brain, especially the hypothalamus, plays a key role in the control of food intake by sensing metabolic signals from peripheral organs and modulating feeding behaviors. To accomplish these important roles, the hypothalamus communicates with other brain areas such as the brainstem and reward-related limbic pathways. The adipocyte-derived hormone leptin and pancreatic β-cell-derived insulin inform adiposity to the hypothalamus. Gut hormones such as cholecystokinin, peptide YY, pancreatic polypeptide, glucagon-like peptide 1, and oxyntomodulin transfer satiety signals to the brain and ghrelin relays hunger signals. The endocannabinoid system and nutrients are also involved in the physiological regulation of food intake. In this article, we briefly review physiological mechanisms of appetite regulation.
-
Citations
Citations to this article as recorded by- Regulation of glycose and lipid metabolism and application based on the colloidal nutrition science properties of konjac glucomannan: A comprehensive review
Pengkui Xia, Ying Zheng, Li Sun, Wenxin Chen, Longchen Shang, Jing Li, Tao Hou, Bin Li
Carbohydrate Polymers.2024; 331: 121849. CrossRef - Weight Regain after Metabolic Surgery: Beyond the Surgical Failure
Juan Salazar, Pablo Duran, Bermary Garrido, Heliana Parra, Marlon Hernández, Clímaco Cano, Roberto Añez, Henry García-Pacheco, Gabriel Cubillos, Neidalis Vasquez, Maricarmen Chacin, Valmore Bermúdez
Journal of Clinical Medicine.2024; 13(4): 1143. CrossRef - Thylakoid supplementation and hunger and fullness perception: a systematic review and dose-response meta-analysis of randomized controlled trials
Negin Nikrad, Mehdi Ghaffari Sarghein, Mahdieh Abbasalizad Farhangi
Nutrition Reviews.2024;[Epub] CrossRef - Stomach clusterin as a gut-derived feeding regulator
Cherl NamKoong, Bohye Kim, Ji Hee Yu, Byung Soo Youn, Hanbin Kim, Evonne Kim, So Young Gil, Gil Myoung Kang, Chan Hee Lee, Young-Bum Kim, Kyeong-Han Park, Min-Seon Kim, Obin Kwon
BMB Reports.2024; 57(3): 149. CrossRef - Anorexigenic neuropeptides as anti-obesity and neuroprotective agents: exploring the neuroprotective effects of anorexigenic neuropeptides
Veronika Strnadová, Andrea Pačesová, Vilém Charvát, Zuzana Šmotková, Blanka Železná, Jaroslav Kuneš, Lenka Maletínská
Bioscience Reports.2024;[Epub] CrossRef - The non-conventional edible plant foroba (Parkia biglobosa) has anti-obesity effect, improves lipid peroxidation and reverses colon and hippocampal lesions in healthy and obese rats
Mirela Gouveia-Nhanca, Maria Luiza Rolim Bezerra, Kamila Sabino Batista, Rafael Oliveira Pinheiro, Naís Lira Soares, Maria Carolina de Paiva Sousa, Adriano Francisco Alves, Mateus Duarte Ribeiro, Alexandre Sergio Silva, Marciane Magnani, Marcos dos Santos
Journal of Functional Foods.2023; 108: 105745. CrossRef - Aberrant bone marrow-derived microglia in the hypothalamus may dysregulate appetite in diabetes
Miwako Katagi, Yuki Nakae, Junko Okano, Kazunori Fujino, Tomoki Tanaka, Itsuko Miyazawa, Natsuko Ohashi, Takahiko Nakagawa, Hideto Kojima
Biochemical and Biophysical Research Communications.2023; 682: 132. CrossRef - Proteins and peptides from vegetable food sources as therapeutic adjuvants for the type 2 diabetes mellitus
Ivan Chan-Zapata, Carlos Sandoval-Castro, Maira Rubí Segura-Campos
Critical Reviews in Food Science and Nutrition.2022; 62(10): 2673. CrossRef - Differential effects of citalopram on the intake of high fat or high carbohydrates diets in female and male rats
Amparo L. De la Fuente-Reynoso, Eliana Barrios De Tomasi, Jorge Juárez
Nutritional Neuroscience.2022; 25(7): 1477. CrossRef - Egzersizin iştah ve iştah hormonları üzerine etkisinin incelenmesi:
PubMed üzerinden yapılmış sistematik derleme
Esmanur Kaya, Şerife Vatansever
Turkish Journal of Sports Medicine.2022; 57(1): 51. CrossRef - Role of Leu72Met of GHRL and Gln223Arg of LEPR Variants on Food Intake, Subjective Appetite, and Hunger-Satiety Hormones
Tania Sanchez-Murguia, Nathaly Torres-Castillo, Lisset Magaña-de la Vega, Saraí Citlalic Rodríguez-Reyes, Wendy Campos-Pérez, Erika Martínez-López
Nutrients.2022; 14(10): 2100. CrossRef - Appetite ratings and ghrelin concentrations in young adults after administration of a balanced meal. Does sex matter?
Alessandro Leone, Ramona De Amicis, Marta Pellizzari, Simona Bertoli, Simone Ravella, Alberto Battezzati
Biology of Sex Differences.2022;[Epub] CrossRef - Interplay between fatty acid desaturase2 (FADS2) rs174583 genetic variant and dietary antioxidant capacity: cardio-metabolic risk factors in obese individuals
Mahdieh Khodarahmi, Parisa Javidzade, Mahdieh Abbasalizad Farhangi, Ahmad Hashemzehi, Houman Kahroba
BMC Endocrine Disorders.2022;[Epub] CrossRef - Appetite-regulating hormones in bipolar disorder: A systematic review and meta-analysis
Błażej Misiak, Krzysztof Kowalski, Bartłomiej Stańczykiewicz, Francesco Bartoli, Giuseppe Carrà, Jerzy Samochowiec, Agnieszka Samochowiec, Dorota Frydecka
Frontiers in Neuroendocrinology.2022; 67: 101013. CrossRef - Association of plasma brain-derived neurotrophic factor levels and frailty in community-dwelling older adults
Eun Roh, Soon Young Hwang, Eyun Song, Min Jeong Park, Hye Jin Yoo, Sei Hyun Baik, Miji Kim, Chang Won Won, Kyung Mook Choi
Scientific Reports.2022;[Epub] CrossRef - Gut Hormones in Health and Obesity: The Upcoming Role of Short Chain Fatty Acids
Habeeb Alhabeeb, Ali AlFaiz, Emad Kutbi, Dayel AlShahrani, Abdullah Alsuhail, Saleh AlRajhi, Nemer Alotaibi, Khalid Alotaibi, Saad AlAmri, Saleh Alghamdi, Naji AlJohani
Nutrients.2021; 13(2): 481. CrossRef - Asprosin ve Glikoz Metabolizması Üzerine Etkileri
M. Gizem KESER, Nurhan ÜNÜSAN
Turkish Journal of Diabetes and Obesity.2021; 5(1): 89. CrossRef - Recent Advances in Understanding Peripheral Taste Decoding I: 2010 to 2020
Jea Hwa Jang, Obin Kwon, Seok Jun Moon, Yong Taek Jeong
Endocrinology and Metabolism.2021; 36(3): 469. CrossRef - Association of increased abdominal adiposity at birth with altered ventral caudate microstructure
Dawn X. P. Koh, Mya Thway Tint, Peter D. Gluckman, Yap Seng Chong, Fabian K. P. Yap, Anqi Qiu, Johan G. Eriksson, Marielle V. Fortier, Patricia P. Silveira, Michael J. Meaney, Ai Peng Tan
International Journal of Obesity.2021; 45(11): 2396. CrossRef - The Crosstalk Between Brain Mediators Regulating Food Intake Behavior in Birds: A Review
Behrouz Rahmani, Elham Ghashghayi, Morteza Zendehdel, Mina Khodadadi, Behnam Hamidi
International Journal of Peptide Research and Therapeutics.2021; 27(4): 2349. CrossRef - Oral Semaglutide, the First Ingestible Glucagon-Like Peptide-1 Receptor Agonist: Could It Be a Magic Bullet for Type 2 Diabetes?
Hwi Seung Kim, Chang Hee Jung
International Journal of Molecular Sciences.2021; 22(18): 9936. CrossRef - Potential Role of Hypothalamic and Plasma Ghrelin in the Feeding Behavior of Obese Type 2 Diabetic Rats with Intraventricular Glucagon-Like Peptide-1 Receptor Agonist Intervention
Ke Lu, Xiaoyan Chen, Xuelian Deng, Juan Long, Jianhua Yan
Obesity Facts.2021; 14(1): 10. CrossRef - Managing obesity through natural polyphenols: A review
Manisha Singh, Thilini Thrimawithana, Ravi Shukla, Benu Adhikari
Future Foods.2020; 1-2: 100002. CrossRef - Neurochemical regulators of food behavior for pharmacological treatment of obesity: current status and future prospects
Gayane Sargis Vardanyan, Hasmik Samvel Harutyunyan, Michail Iosif Aghajanov, Ruben Sargis Vardanyan
Future Medicinal Chemistry.2020; 12(20): 1865. CrossRef - Modulation of feeding behavior and metabolism by dynorphin
Aishwarya Ghule, Ildiko Rácz, Andras Bilkei-Gorzo, Este Leidmaa, Meike Sieburg, Andreas Zimmer
Scientific Reports.2020;[Epub] CrossRef - Possible role of peptide YY (PYY) in the pathophysiology of irritable bowel syndrome (IBS)
Magdy El-Salhy, Jan Gunnar Hatlebakk, Trygve Hausken
Neuropeptides.2020; 79: 101973. CrossRef - Prolactin-releasing peptide increases food intake and affects hypothalamic physiology in Japanese quail (Coturnix japonica)
B.R. McConn, T. Tachibana, E.R. Gilbert, M.A. Cline
Domestic Animal Endocrinology.2020; 72: 106464. CrossRef - Obesity induced by Borna disease virus in rats: key roles of hypothalamic fast-acting neurotransmitters and inflammatory infiltrates
Georg Gosztonyi, Hanns Ludwig, Liv Bode, Moujahed Kao, Manfred Sell, Peter Petrusz, Béla Halász
Brain Structure and Function.2020; 225(5): 1459. CrossRef - D‐methionine improves cisplatin‐induced anorexia and dyspepsia syndrome by attenuating intestinal tryptophan hydroxylase 1 activity and increasing plasma leptin concentration
Yi‐Sin Wong, Meei‐Yn Lin, Pei‐Fen Liu, Jiunn‐Liang Ko, Guan‐Ting Huang, Dom‐Gene Tu, Chu‐Chyn Ou
Neurogastroenterology & Motility.2020;[Epub] CrossRef - Effects of oral, smoked, and vaporized cannabis on endocrine pathways related to appetite and metabolism: a randomized, double-blind, placebo-controlled, human laboratory study
Mehdi Farokhnia, Gray R. McDiarmid, Matthew N. Newmeyer, Vikas Munjal, Osama A. Abulseoud, Marilyn A. Huestis, Lorenzo Leggio
Translational Psychiatry.2020;[Epub] CrossRef - Role of Paraventricular Nucleus in Regulation of Feeding Behaviour and the Design of Intranuclear Neuronal Pathway Communications
Shiba Yousefvand, Farshid Hamidi
International Journal of Peptide Research and Therapeutics.2020; 26(3): 1231. CrossRef - Self-Reported Eating Speed and Incidence of Gestational Diabetes Mellitus: the Japan Environment and Children’s Study
Jia-Yi Dong, Satoyo Ikehara, Takashi Kimura, Meishan Cui, Yoko Kawanishi, Tadashi Kimura, Kimiko Ueda, Hiroyasu Iso
Nutrients.2020; 12(5): 1296. CrossRef - Effects of a high-fat-diet supplemented with probiotics and ω3-fatty acids on appetite regulatory neuropeptides and neurotransmitters in a pig model
D. Valent, L. Arroyo, E. Fàbrega, M. Font-i-Furnols, M. Rodríguez-Palmero, J.A. Moreno-Muñoz, J. Tibau, A. Bassols
Beneficial Microbes.2020; 11(4): 347. CrossRef - Electro-Acupuncture Alleviates Cisplatin-Induced Anorexia in Rats by Modulating Ghrelin and Monoamine Neurotransmitters
Ji Yun Baek, Tuy An Trinh, Wonsang Huh, Ji Hoon Song, Hyun Young Kim, Juhee Lim, Jinhee Kim, Hyun Jin Choi, Tae-Hun Kim, Ki Sung Kang
Biomolecules.2019; 9(10): 624. CrossRef - Interleukin-6 Expression by Hypothalamic Microglia in Multiple Inflammatory Contexts: A Systematic Review
Vanessa C. D. Bobbo, Carlos P. Jara, Natália F. Mendes, Joseane Morari, Lício A. Velloso, Eliana P. Araújo
BioMed Research International.2019; 2019: 1. CrossRef - Abnormalities in Glucose Metabolism, Appetite-Related Peptide Release, and Pro-inflammatory Cytokines Play a Central Role in Appetite Disorders in Peritoneal Dialysis
Lorena Avila-Carrasco, Mario A. Pavone, Elena González, Álvaro Aguilera-Baca, Rafael Selgas, Gloria del Peso, Secundino Cigarran, Manuel López-Cabrera, Abelardo Aguilera
Frontiers in Physiology.2019;[Epub] CrossRef - Branched chain amino acids stimulate gut satiety hormone cholecystokinin secretion through activation of the umami taste receptor T1R1/T1R3 using an in vitro porcine jejunum model
Min Tian, Jinghui Heng, Hanqing Song, Yufeng Zhang, Fang Chen, Wutai Guan, Shihai Zhang
Food & Function.2019; 10(6): 3356. CrossRef - Multi-Omic Biological Age Estimation and Its Correlation With Wellness and Disease Phenotypes: A Longitudinal Study of 3,558 Individuals
John C Earls, Noa Rappaport, Laura Heath, Tomasz Wilmanski, Andrew T Magis, Nicholas J Schork, Gilbert S Omenn, Jennifer Lovejoy, Leroy Hood, Nathan D Price, David Le Couteur
The Journals of Gerontology: Series A.2019; 74(Supplement): S52. CrossRef - The impact of sugar consumption on stress driven, emotional and addictive behaviors
Angela Jacques, Nicholas Chaaya, Kate Beecher, Syed Aoun Ali, Arnauld Belmer, Selena Bartlett
Neuroscience & Biobehavioral Reviews.2019; 103: 178. CrossRef - Leptin Signaling in the Control of Metabolism and Appetite: Lessons from Animal Models
Alberto A. Barrios-Correa, José A. Estrada, Irazú Contreras
Journal of Molecular Neuroscience.2018; 66(3): 390. CrossRef - Role of paraventricular hypothalamic dopaminergic D1 receptors in food intake regulation of food-deprived rats
Zahra. Mirmohammadsadeghi, Masoud. Shareghi Brojeni, Abbas. Haghparast, Afsaneh. Eliassi
European Journal of Pharmacology.2018; 818: 43. CrossRef - Integrating Thyroid Hormone Signaling in Hypothalamic Control of Metabolism: Crosstalk Between Nuclear Receptors
Soumaya Kouidhi, Marie-Stéphanie Clerget-Froidevaux
International Journal of Molecular Sciences.2018; 19(7): 2017. CrossRef - Review article: Role of satiety hormones in anorexia induction by Trichothecene mycotoxins
Chloé Terciolo, Marc Maresca, Philippe Pinton, Isabelle P. Oswald
Food and Chemical Toxicology.2018; 121: 701. CrossRef - Obezite ve Ghrelin/Leptin İlişkisi
Aliye Sağkan Öztürk, Abdullah ARPACI
Mustafa Kemal Üniversitesi Tıp Dergisi.2018; 9(35): 136. CrossRef - Overexpression of Wild-Type Human Alpha-Synuclein Causes Metabolism Abnormalities in Thy1-aSYN Transgenic Mice
Elodie Cuvelier, Mathieu Méquinion, Coline Leghay, William Sibran, Aliçia Stievenard, Alessia Sarchione, Marie-Amandine Bonte, Christel Vanbesien-Mailliot, Odile Viltart, Kevin Saitoski, Emilie Caron, Alexandra Labarthe, Thomas Comptdaer, Pierre Semaille,
Frontiers in Molecular Neuroscience.2018;[Epub] CrossRef - Aetiology of eating behaviours: A possible mechanism to understand obesity development in early childhood
Nikki Boswell, Rebecca Byrne, Peter S.W. Davies
Neuroscience & Biobehavioral Reviews.2018; 95: 438. CrossRef - Clinical Phenotype of Depression Affects Interleukin-6 Synthesis
Łukasz Zadka, Piotr Dzięgiel, Michał Kulus, Marcin Olajossy
Journal of Interferon & Cytokine Research.2017; 37(6): 231. CrossRef - Altered Adipogenesis in Zebrafish Larvae Following High Fat Diet and Chemical Exposure Is Visualised by Stimulated Raman Scattering Microscopy
Marjo den Broeder, Miriam Moester, Jorke Kamstra, Peter Cenijn, Valentina Davidoiu, Leonie Kamminga, Freek Ariese, Johannes de Boer, Juliette Legler
International Journal of Molecular Sciences.2017; 18(4): 894. CrossRef - SIFamide Translates Hunger Signals into Appetitive and Feeding Behavior in Drosophila
Carlotta Martelli, Ulrike Pech, Simon Kobbenbring, Dennis Pauls, Britta Bahl, Mirjam Vanessa Sommer, Atefeh Pooryasin, Jonas Barth, Carmina Warth Perez Arias, Chrystalleni Vassiliou, Abud Jose Farca Luna, Haiko Poppinga, Florian Gerhard Richter, Christian
Cell Reports.2017; 20(2): 464. CrossRef - Effect of Mulberry Extract on the Lipid Profile and Liver Function in Mice Fed a High Fat Diet
Kyung-Soon Choi, Yong-Hwan Kim, Kyung-Ok Shin
The Korean Journal of Food And Nutrition.2016; 29(3): 411. CrossRef - Helicobacter pylori Infection in Children: Nutritional Status and Associations with Serum Leptin, Ghrelin, and IGF‐1 Levels
Gulin Erdemir, Tanju Basarir Ozkan, Taner Ozgur, Derya Altay, Sinan Cavun, Guher Goral
Helicobacter.2016; 21(4): 317. CrossRef - Dietary Capsaicin Protects Cardiometabolic Organs from Dysfunction
Fang Sun, Shiqiang Xiong, Zhiming Zhu
Nutrients.2016; 8(5): 174. CrossRef - Effects of Short-Term Exenatide Treatment on Regional Fat Distribution, Glycated Hemoglobin Levels, and Aortic Pulse Wave Velocity of Obese Type 2 Diabetes Mellitus Patients
Ju-Young Hong, Keun-Young Park, Byung-Joon Kim, Won-Min Hwang, Dong-Ho Kim, Dong-Mee Lim
Endocrinology and Metabolism.2016; 31(1): 80. CrossRef - The role of the neuropeptide Y (NPY) family in the pathophysiology of inflammatory bowel disease (IBD)
Magdy El-Salhy, Trygve Hausken
Neuropeptides.2016; 55: 137. CrossRef - Proactive and Progressive Approaches in Managing Obesity
Robert H. Eckel, Harold E. Bays, Samuel Klein, Deborah Bade Horn
Postgraduate Medicine.2016; 128(sup1): 21. CrossRef - The role of food intake regulating peptides in cardiovascular regulation
B. Mikulášková, L. Maletínská, J. Zicha, J. Kuneš
Molecular and Cellular Endocrinology.2016; 436: 78. CrossRef - Altered gut and adipose tissue hormones in overweight and obese individuals: cause or consequence?
M E J Lean, D Malkova
International Journal of Obesity.2016; 40(4): 622. CrossRef - Expression of NUCB2/nesfatin-1 in the taste buds of rats
Xun Cao, Xiao Zhou, Yang Cao, Xiao-Min Liu, Li-Hong Zhou
Endocrine Journal.2016; 63(1): 37. CrossRef - Brain Regulation of Energy Metabolism
Eun Roh, Min-Seon Kim
Endocrinology and Metabolism.2016; 31(4): 519. CrossRef - Stopped-Flow Studies of the Reduction of the Copper Centers Suggest a Bifurcated Electron Transfer Pathway in Peptidylglycine Monooxygenase
Shefali Chauhan, Parisa Hosseinzadeh, Yi Lu, Ninian J. Blackburn
Biochemistry.2016; 55(13): 2008. CrossRef - Potential role of bioactive compounds of Phaseolus vulgaris L. on lipid-lowering mechanisms
Aurea K. Ramírez-Jiménez, Rosalía Reynoso-Camacho, M. Elizabeth Tejero, Fabiola León-Galván, Guadalupe Loarca-Piña
Food Research International.2015; 76: 92. CrossRef - Recent developments in the pathophysiology of irritable bowel syndrome
Magdy El-Salhy
World Journal of Gastroenterology.2015; 21(25): 7621. CrossRef - Lifestyle Changes Followed by Bariatric Surgery Lower Inflammatory Markers and the Cardiovascular Risk Factors C3 and C4
Torunn Kristin Nestvold, Erik Waage Nielsen, Judith Krey Ludviksen, Hilde Fure, Anne Landsem, Knut Tore Lappegård
Metabolic Syndrome and Related Disorders.2015; 13(1): 29. CrossRef - Diet in irritable bowel syndrome
Magdy El-Salhy, Doris Gundersen
Nutrition Journal.2015;[Epub] CrossRef - 3p22.1p21.31 microdeletion identifies CCK as Asperger syndrome candidate gene and shows the way for therapeutic strategies in chromosome imbalances
Ivan Y. Iourov, Svetlana G. Vorsanova, Victoria Y. Voinova, Yuri B. Yurov
Molecular Cytogenetics.2015;[Epub] CrossRef - The effect of slow spaced eating on hunger and satiety in overweight and obese patients with type 2 diabetes mellitus
Theodoros Angelopoulos, Alexander Kokkinos, Christos Liaskos, Nicholas Tentolouris, Kleopatra Alexiadou, Alexander Dimitri Miras, Iordanis Mourouzis, Despoina Perrea, Constantinos Pantos, Nicholas Katsilambros, Stephen R Bloom, Carel Wynard le Roux
BMJ Open Diabetes Research & Care.2014; 2(1): e000013. CrossRef - Bisphenol A is related to circulating levels of adiponectin, leptin and ghrelin, but not to fat mass or fat distribution in humans
Monika Rönn, Lars Lind, Jan Örberg, Joel Kullberg, Stefan Söderberg, Anders Larsson, Lars Johansson, Håkan Ahlström, P. Monica Lind
Chemosphere.2014; 112: 42. CrossRef - The modulatory role of alpha-melanocyte stimulating hormone administered spinally in the regulation of blood glucose level in d-glucose-fed and restraint stress mouse models
Yun-Beom Sim, Soo-Hyun Park, Sung-Su Kim, Su-Min Lim, Jun-Sub Jung, Hong-Won Suh
Neuropeptides.2014; 48(4): 207. CrossRef - The forgotten members of the glucagon family
Dominique Bataille, Stéphane Dalle
Diabetes Research and Clinical Practice.2014; 106(1): 1. CrossRef - Incretin mimetics as pharmacologic tools to elucidate and as a new drug strategy to treat traumatic brain injury
Nigel H. Greig, David Tweedie, Lital Rachmany, Yazhou Li, Vardit Rubovitch, Shaul Schreiber, Yung-Hsiao Chiang, Barry J. Hoffer, Jonathan Miller, Debomoy K. Lahiri, Kumar Sambamurti, Robert E. Becker, Chaim G. Pick
Alzheimer's & Dementia.2014;[Epub] CrossRef - Fatty acid analysis and regulatory effects of citron (Citrus junosSieb. ex TANAKA) seed oil on nitric oxide production, lipid accumulation, and leptin secretion
Tae Woo Kim, Kyoung Kon Kim, Yun Hwan Kang, Dae Jung Kim, Myeon Choe
Journal of Nutrition and Health.2014; 47(4): 221. CrossRef - Analysis of Pine Nut Oil Composition and Its Effects on Obesity
Kyoung Kon Kim, Yun Hwan Kang, Dae Jung Kim, Tae Woo Kim, Myeon Choe
Korean Journal of Food Science and Technology.2014; 46(5): 630. CrossRef - Anti-obesity effects of KR-66195, a synthetic DPP-IV inhibitor, in diet-induced obese mice and obese-diabetic ob/ob mice
Eun Young Lee, Yeon Wook Kim, Hyunhee Oh, Cheol Soo Choi, Jin Hee Ahn, Byung-Wan Lee, Eun Seok Kang, Bong Soo Cha, Hyun Chul Lee
Metabolism.2014; 63(6): 793. CrossRef - Position and Length of Fatty Acids Strongly Affect Receptor Selectivity Pattern of Human Pancreatic Polypeptide Analogues
Veronika Mäde, Kathrin Bellmann‐Sickert, Anette Kaiser, Jens Meiler, Annette G. Beck‐Sickinger
ChemMedChem.2014; 9(11): 2463. CrossRef - Regulation of food intake after surgery and the gut brain axis
Nilanjana Tewari, Sherif Awad, Dileep N. Lobo
Current Opinion in Clinical Nutrition and Metabolic Care.2013; 16(5): 569. CrossRef - Effect of ambient temperature during acute aerobic exercise on short-term appetite, energy intake, and plasma acylated ghrelin in recreationally active males
Lucy K. Wasse, James A. King, David J. Stensel, Caroline Sunderland
Applied Physiology, Nutrition, and Metabolism.2013; 38(8): 905. CrossRef - Peripheral Pathways in the Food-Intake Control towards the Adipose-Intestinal Missing Link
Hugo Mendieta Zerón, Ma. Victoria Domínguez García, María del Socorro Camarillo Romero, Miriam V. Flores-Merino
International Journal of Endocrinology.2013; 2013: 1. CrossRef - Alteration of sweet taste in high-fat diet induced obese rats after 4 weeks treatment with exenatide
Xiao-juan Zhang, Yu-qing Wang, Yang Long, Lei Wang, Yun Li, Fa-bao Gao, Hao-ming Tian
Peptides.2013; 47: 115. CrossRef - Decrease of Obesity by Allantoin via Imidazoline I1-Receptor Activation in High Fat Diet-Fed Mice
Hsien-Hui Chung, Kung Shing Lee, Juei-Tang Cheng
Evidence-Based Complementary and Alternative Medicine.2013; 2013: 1. CrossRef - Hunger Hormone Profile Monitoring after Gastroplication in an Adolescent
Valeria Calcaterra, Gloria Pelizzo, Ghassan Nakib, Daniela Larizza, Maria Luisa Fonte, Mara De Amici, Hellas Cena
Hormone Research in Paediatrics.2013; 80(3): 213. CrossRef
- Regulation of glycose and lipid metabolism and application based on the colloidal nutrition science properties of konjac glucomannan: A comprehensive review
- Intracerebroventricular Injection of Metformin Induces Anorexia in Rats
- Chang Koo Lee, Yoon Jung Choi, So Young Park, Jong Yeon Kim, Kyu Chang Won, Yong Woon Kim
- Diabetes Metab J. 2012;36(4):293-299. Published online August 20, 2012
- DOI: https://doi.org/10.4093/dmj.2012.36.4.293
- 4,452 View
- 42 Download
- 27 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Metformin, an oral biguanide insulin-sensitizing agent, is well known to decrease appetite. Although there is evidence that metformin could affect the brain directly, the exact mechanism is not yet known.
Methods To evaluate whether metformin induces anorexia via the hypothalamus, various concentrations of metformin were injected into the lateral ventricle of rats through a chronically implanted catheter and food intake was measured for 24 hours. The hypothalamic neuropeptides associated with regulation of food intake were also analyzed following 1 hour of intracerebroventricular (ICV) injections of metformin.
Results An ICV injection of metformin decreased food intake in a dose-dependent manner in unrestrained conscious rats. Hypothalamic phosphorylated AMP-activated protein kinase (pAMPK) increased by 3 µg with metformin treatment, but there was no further increase in pAMPK with increases in metformin dosage. The hypothalamic phosphorylated signal transducer and activator of transcription 3 (pSTAT3) increased by 3 µg with metformin treatment, but, there was no further increase in pSTAT3 level following increases of metformin dosage. Hypothalamic proopiomelanocortin was elevated with metformin treatment, while neuropeptide Y was not significantly changed.
Conclusion Our results suggest that metformin induces anorexia via direct action in the hypothalamus and the increase in pSTAT3, at least in part, is involved in the process. However, hypothalamic pAMPK appears not to contribute to metformin-induced appetite reduction in normal rats. Further studies exploring new pathways connecting metformin and feeding regulation are needed.
-
Citations
Citations to this article as recorded by- Metabolic and Metabolomic Effects of Metformin in Murine Model of Pulmonary Adenoma Formation
Andrew C. Elton, Vannesa Cedarstrom, Arman Quraishi, Beverly Wuertz, Kevin Murray, Todd W. Markowski, Donna Seabloom, Frank G. Ondrey
Nutrition and Cancer.2023; 75(3): 1014. CrossRef - Steroidogenic Effect of Luteinizing Hormone Receptor Agonists and Metformin in Male Rats with Androgenic Deficiency Caused by Diet-Induced Obesity
A. A. Bakhtyukov, K. V. Derkach, I. A. Lebedev, V. N. Sorokoumov, A. O. Shpakov
Journal of Evolutionary Biochemistry and Physiology.2023; 59(5): 1810. CrossRef - Steroidogenic Effect of Luteinizing Hormone Receptor Agonists and Metformin in Male Rats with Androgenic Deficiency Caused by Diet-Induced Obesity
A. A. Bakhtyukov, K. V. Derkach, I. A. Lebedev, V. N. Sorokoumov, A. O. Shpakov
Российский физиологический журнал им И М Сеченова.2023; 109(10): 1414. CrossRef - Metformin in nucleus accumbens core reduces cue‐induced cocaine seeking in male and female rats
Amy Chan, Alexis Willard, Sarah Mulloy, Noor Ibrahim, Allegra Sciaccotta, Mark Schonfeld, Sade M. Spencer
Addiction Biology.2022;[Epub] CrossRef - Knockdown of Endogenous Nucb2/Nesfatin-1 in the PVN Leads to
Obese-Like Phenotype and Abolishes the Metformin- and Stress-Induced Thermogenic
Response in Rats
Daniel Stephan, Natalie Taege, Riccardo Dore, Julica Folberth, Olaf Jöhren, Markus Schwaninger, Hendrik Lehnert, Carla Schulz
Hormone and Metabolic Research.2022; 54(11): 768. CrossRef - Modulation of hypothalamic AMPK phosphorylation by olanzapine controls energy balance and body weight
Vitor Ferreira, Cintia Folgueira, Maria Guillén, Pablo Zubiaur, Marcos Navares, Assel Sarsenbayeva, Pilar López-Larrubia, Jan W. Eriksson, Maria J. Pereira, Francisco Abad-Santos, Guadalupe Sabio, Patricia Rada, Ángela M. Valverde
Metabolism.2022; 137: 155335. CrossRef - Metformin acts on the gut-brain axis to ameliorate antipsychotic-induced metabolic dysfunction
Xiaorong Wang, Huimin Huang, Yiyi Zhu, Shaoli Li, Peifen Zhang, Jiajun Jiang, Caixi Xi, Lingling Wu, Xingle Gao, Yaoyang Fu, Danhua Zhang, Yiqing Chen, Shaohua Hu, Jianbo Lai
BioScience Trends.2021; 15(5): 321. CrossRef - Therapeutic effect of treatment with metformin and/or 4-hydroxychalcone in male Wistar rats with nonalcoholic fatty liver disease
Selene de Jesús Acosta-Cota, Elsa Maribel Aguilar-Medina, Rosalío Ramos-Payán, José Guadalupe Rendón Maldonado, José Geovanni Romero-Quintana, Julio Montes-Avila, Juan I. Sarmiento-Sánchez, Carolina Gabriela Plazas-Guerrero, Marcela J. Vergara-Jiménez, Ar
European Journal of Pharmacology.2019; 863: 172699. CrossRef - The evidence of metabolic-improving effect of metformin in Ay/a mice with genetically-induced melanocortin obesity and the contribution of hypothalamic mechanisms to this effect
Kira Derkach, Irina Zakharova, Inna Zorina, Andrey Bakhtyukov, Irina Romanova, Liubov Bayunova, Alexander Shpakov, Guillermo López Lluch
PLOS ONE.2019; 14(3): e0213779. CrossRef - Effect of Metformin on Antipsychotic-Induced Metabolic Dysfunction: The Potential Role of Gut-Brain Axis
Chao Luo, Xu Wang, Hanxue Huang, Xiaoyuan Mao, Honghao Zhou, Zhaoqian Liu
Frontiers in Pharmacology.2019;[Epub] CrossRef - Metformin alters signaling induced crosstalk and homeostasis in the carcinogenesis paradigm “Epistemology of the origin of cancer”
Björn L.D.M. Brücher, Ijaz S. Jamall, Obul R. Bandapalli
4open.2019; 2: 12. CrossRef - Melatonin potentiates the effects of metformin on glucose metabolism and food intake in high‐fat‐fed rats
Rosana F. Dantas‐Ferreira, Helene Raingard, Stephanie Dumont, Carole Schuster‐Klein, Beatrice Guardiola‐Lemaitre, Paul Pevet, Etienne Challet
Endocrinology, Diabetes & Metabolism.2018;[Epub] CrossRef -
Molecular Mechanisms of the Effects of Metformin on the Functional Activity of Brain Neurons
A. O. Shpakov, K. V. Derkach
Neuroscience and Behavioral Physiology.2018; 48(8): 969. CrossRef - Effect of metformin/irinotecan-loaded poly-lactic-co-glycolic acid nanoparticles on glioblastoma: in vitro and in vivo studies
Ali Taghizadehghalehjoughi, Ahmet Hacimuftuoglu, Meltem Cetin, Afife Busra Ugur, Bianca Galateanu, Yaroslav Mezhuev, Ufuk Okkay, Numan Taspinar, Mehmet Taspinar, Abdullah Uyanik, Betul Gundogdu, Maryam Mohammadzadeh, Kemal Alp Nalci, Polychronis Stivaktak
Nanomedicine.2018; 13(13): 1595. CrossRef - Effect of Betahistine and Metformin on Antipsychotic-Induced Weight Gain: An Analysis of Two Clinical Trials
Dongyu Kang, Zhihui Jing, Ranran Li, Gangrui Hei, Tiannan Shao, Li Li, Mengxi Sun, Ye Yang, Ying Wang, Xiaoyi Wang, Yujun Long, Xiansheng Huang, Renrong Wu
Frontiers in Psychiatry.2018;[Epub] CrossRef - Metformin: not only per os
Lev M. Berstein
Expert Review of Endocrinology & Metabolism.2018; 13(2): 63. CrossRef - МЕТАБОЛИЧЕСКИЕ ПОКАЗАТЕЛИ И ФУНКЦИОНАЛЬНОЕ СОСТОЯНИЕ СИГНАЛЬНЫХ СИСТЕМ ГИПОТАЛАМУСА И ВЛИЯНИЕ НА НИХ МЕТФОРМИНА У МЫШЕЙ С МУТАЦИЕЙ AY/A, ГЕНЕТИЧЕСКИ ПРЕДРАСПОЛОЖЕННЫХ К ОЖИРЕНИЮ, "Доклады Академии наук"
К.В. Деркач, И.О. Захарова, И.В. Романова, И. И. Зорина, А.Л. Михрина, А.О. Шпаков
Доклады Академии Наук.2017; (4): 488. CrossRef - Beneficial effects of metformin on energy metabolism and visceral fat volume through a possible mechanism of fatty acid oxidation in human subjects and rats
Ichiro Tokubuchi, Yuji Tajiri, Shimpei Iwata, Kento Hara, Nobuhiko Wada, Toshihiko Hashinaga, Hitomi Nakayama, Hiroharu Mifune, Kentaro Yamada, M. Faadiel Essop
PLOS ONE.2017; 12(2): e0171293. CrossRef - Metabolic parameters and functional state of hypothalamic signaling systems in AY/a mice with genetic predisposition to obesity and the effect of metformin
K. V. Derkach, I. O. Zakharova, I. V. Romanova, I. I. Zorina, A. L. Mikhrina, A. O. Shpakov
Doklady Biochemistry and Biophysics.2017; 477(1): 377. CrossRef - Prolonged metformin treatment leads to reduced transcription of Nrf2 and neurotrophic factors without cognitive impairment in older C57BL/6J mice
Joanne S. Allard, Evelyn J. Perez, Koji Fukui, Priscilla Carpenter, Donald K. Ingram, Rafael de Cabo
Behavioural Brain Research.2016; 301: 1. CrossRef - Intracerebroventricular Metformin Decreases Body Weight But Has Pro-oxidant Effects and Decreases Survival
Luis Valmor Portela, Jussania Gnoatto, Andressa Wigner Brochier, Clarissa Branco Haas, Adriano Martimbianco de Assis, Afonso Kopczynski de Carvalho, Gisele Hansel, Eduardo Rigon Zimmer, Jean Pierre Oses, Alexandre Pastoris Muller
Neurochemical Research.2015; 40(3): 514. CrossRef - Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway
Young Mi Song, Yong-ho Lee, Ji-Won Kim, Dong-Sik Ham, Eun-Seok Kang, Bong Soo Cha, Hyun Chul Lee, Byung-Wan Lee
Autophagy.2015; 11(1): 46. CrossRef - Activation of AMP-activated protein kinase by metformin protects against global cerebral ischemia in male rats: Interference of AMPK/PGC-1α pathway
Ghorbangol Ashabi, Fariba Khodagholi, Leila Khalaj, Mahdi Goudarzvand, Masoumeh Nasiri
Metabolic Brain Disease.2014; 29(1): 47. CrossRef - Acute oral metformin enhances satiation and activates brainstem nesfatinergic neurons
Thaïs Rouquet, Pierre Clément, Stéphanie Gaigé, Catherine Tardivel, Julien Roux, Michel Dallaporta, Bruno Bariohay, Jean-Denis Troadec, Bruno Lebrun
Obesity.2014; : n/a. CrossRef - Metformin—mode of action and clinical implications for diabetes and cancer
Ida Pernicova, Márta Korbonits
Nature Reviews Endocrinology.2014; 10(3): 143. CrossRef - Effects of metformin on weight loss
Steven K. Malin, Sangeeta R. Kashyap
Current Opinion in Endocrinology, Diabetes & Obesity.2014; 21(5): 323. CrossRef - The effect of ghrelin on MK-801 induced memory impairment in rats
Fatemeh Goshadrou, Mojtaba Kermani, Abdolaziz Ronaghi, Samad Sajjadi
Peptides.2013; 44: 60. CrossRef
- Metabolic and Metabolomic Effects of Metformin in Murine Model of Pulmonary Adenoma Formation
- ATP-Sensitive Potassium Channel-Deficient Mice Show Hyperphagia but Are Resistant to Obesity
- Yeul Bum Park, Yun Jung Choi, So Young Park, Jong Yeon Kim, Seong Ho Kim, Dae Kyu Song, Kyu Chang Won, Yong Woon Kim
- Diabetes Metab J. 2011;35(3):219-225. Published online June 30, 2011
- DOI: https://doi.org/10.4093/dmj.2011.35.3.219
- 3,538 View
- 31 Download
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The hypothalamus, the center for body weight regulation, can sense changes in blood glucose level based on ATP-sensitive potassium (KATP) channels in the hypothalamic neurons. We hypothesized that a lack of glucose sensing in the hypothalamus affects the regulations of appetite and body weight.
Methods To evaluate this hypothesis, the responses to glucose loading and high fat feeding for eight weeks were compared in Kir6.2 knock-out (KO) mice and control C57BL/6 mice, because Kir6.2 is a key component of the KATP channel.
Results The hypothalamic neuropeptide Y (NPY) analyzed one hour after glucose injection was suppressed in C57BL/6 mice, but not in Kir6.2 KO mice, suggesting a blunted hypothalamic response to glucose in Kir6.2 KO mice. The hypothalamic NPY expression at a fed state was elevated in Kir6.2 KO mice and was accompanied with hyperphagia. However, the retroperitoneal fat mass was markedly decreased in Kir6.2 KO mice compared to that in C57BL/6 mice. Moreover, the body weight and visceral fat following eight weeks of high fat feeding in Kir6.2 KO mice were not significantly different from those in control diet-fed Kir6.2 KO mice, while body weight and visceral fat mass were elevated due to high fat feeding in C57BL/6 mice.
Conclusion These results suggested that Kir6.2 KO mice showed a blunted hypothalamic response to glucose loading and elevated hypothalamic NPY expression accompanied with hyperphagia, while visceral fat mass was decreased, suggesting resistance to diet-induced obesity. Further study is needed to explain this phenomenon.
-
Citations
Citations to this article as recorded by- 17β-estradiol promotes acute refeeding in hungry mice via membrane-initiated ERα signaling
Kaifan Yu, Yanlin He, Ilirjana Hyseni, Zhou Pei, Yongjie Yang, Pingwen Xu, Xing Cai, Hesong Liu, Na Qu, Hailan Liu, Yang He, Meng Yu, Chen Liang, Tingting Yang, Julia Wang, Pierre Gourdy, Jean-Francois Arnal, Francoise Lenfant, Yong Xu, Chunmei Wang
Molecular Metabolism.2020; 42: 101053. CrossRef - Brain Glucose-Sensing Mechanism and Energy Homeostasis
A. J. López-Gambero, F. Martínez, K. Salazar, M. Cifuentes, F. Nualart
Molecular Neurobiology.2019; 56(2): 769. CrossRef - The involvement of purinergic signalling in obesity
Geoffrey Burnstock, Daniela Gentile
Purinergic Signalling.2018; 14(2): 97. CrossRef - High‐fat‐diet‐induced remission of diabetes in a subset of KATP‐GOF insulin‐secretory‐deficient mice
Zihan Yan, Zeenat A. Shyr, Manuela Fortunato, Alecia Welscher, Mariana Alisio, Michael Martino, Brian N. Finck, Hannah Conway, Maria S. Remedi
Diabetes, Obesity and Metabolism.2018; 20(11): 2574. CrossRef - Hypothalamic arcuate nucleus glucokinase regulates insulin secretion and glucose homeostasis
Yue Ma, Risheka Ratnasabapathy, Chioma Izzi‐Engbeaya, Marie‐Sophie Nguyen‐Tu, Errol Richardson, Sufyan Hussain, Ivan De Backer, Christopher Holton, Mariana Norton, Gaelle Carrat, Blanche Schwappach, Guy A. Rutter, Waljit S. Dhillo, James Gardiner
Diabetes, Obesity and Metabolism.2018; 20(9): 2246. CrossRef - Overexpression of WNK1 in POMC-expressing neurons reduces weigh gain via WNK4-mediated degradation of Kir6.2
Woo Young Chung, Jung Woo Han, Woon Heo, Min Goo Lee, Joo Young Kim
Molecular and Cellular Biochemistry.2018; 447(1-2): 165. CrossRef - Insights into the role of neuronal glucokinase
Ivan De Backer, Sufyan S. Hussain, Stephen R. Bloom, James V. Gardiner
American Journal of Physiology-Endocrinology and Metabolism.2016; 311(1): E42. CrossRef - Obesogenic and Diabetogenic Effects of High-Calorie Nutrition Require Adipocyte BK Channels
Julia Illison, Lijun Tian, Heather McClafferty, Martin Werno, Luke H. Chamberlain, Veronika Leiss, Antonia Sassmann, Stefan Offermanns, Peter Ruth, Michael J. Shipston, Robert Lukowski
Diabetes.2016; 65(12): 3621. CrossRef -
ABCC8 R1420H Loss-of-Function Variant in a Southwest American Indian Community: Association With Increased Birth Weight and Doubled Risk of Type 2 Diabetes
Leslie J. Baier, Yunhua Li Muller, Maria Sara Remedi, Michael Traurig, Paolo Piaggi, Gregory Wiessner, Ke Huang, Alyssa Stacy, Sayuko Kobes, Jonathan Krakoff, Peter H. Bennett, Robert G. Nelson, William C. Knowler, Robert L. Hanson, Colin G. Nichols, Clif
Diabetes.2015; 64(12): 4322. CrossRef - Glucokinase activity in the arcuate nucleus regulates glucose intake
Syed Hussain, Errol Richardson, Yue Ma, Christopher Holton, Ivan De Backer, Niki Buckley, Waljit Dhillo, Gavin Bewick, Shuai Zhang, David Carling, Steve Bloom, James Gardiner
Journal of Clinical Investigation.2015; 125(1): 337. CrossRef - Dissecting the Etiology of Type 2 Diabetes in the Pima Indian Population
Ewan R. Pearson
Diabetes.2015; 64(12): 3993. CrossRef - Ocular Hypotensive Effects of the ATP-Sensitive Potassium Channel Opener Cromakalim in Human and Murine Experimental Model Systems
Uttio Roy Chowdhury, Cindy K. Bahler, Bradley H. Holman, Peter I. Dosa, Michael P. Fautsch, Ted S Acott
PLOS ONE.2015; 10(11): e0141783. CrossRef - Differential gene expression pattern in hypothalamus of chickens during fasting-induced metabolic reprogramming: Functions of glucose and lipid metabolism in the feed intake of chickens
Xin-Ling Fang, Xiao-Tong Zhu, Sheng-Feng Chen, Zhi-Qi Zhang, Qing-Jie Zeng, Lin Deng, Jian-Long Peng, Jian-Jian Yu, Li-Na Wang, Song-Bo Wang, Ping Gao, Qing-Yan Jiang, Gang Shu
Poultry Science.2014; 93(11): 2841. CrossRef - Combined Treatment of Betulinic Acid, a PTP1B Inhibitor, with Orthosiphon stamineus Extract Decreases Body Weight in High-Fat–Fed Mice
Yoon-Jung Choi, So-Young Park, Jong-Yeon Kim, Kyu-Chang Won, Bo-Ra Kim, Jong-Keun Son, Seung-Ho Lee, Yong-Woon Kim
Journal of Medicinal Food.2013; 16(1): 2. CrossRef
- 17β-estradiol promotes acute refeeding in hungry mice via membrane-initiated ERα signaling

 KDA
KDA
 First
First Prev
Prev





