
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
- N6-Methyladenosine Methyltransferase METTL3 Alleviates Diabetes-Induced Testicular Damage through Modulating TUG1/Clusterin Axis
- Yuan Tian, Yue-Hai Xiao, Chao Sun, Bei Liu, Fa Sun
- Diabetes Metab J. 2023;47(2):287-300. Published online January 19, 2023
- DOI: https://doi.org/10.4093/dmj.2021.0306
- 2,201 View
- 153 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
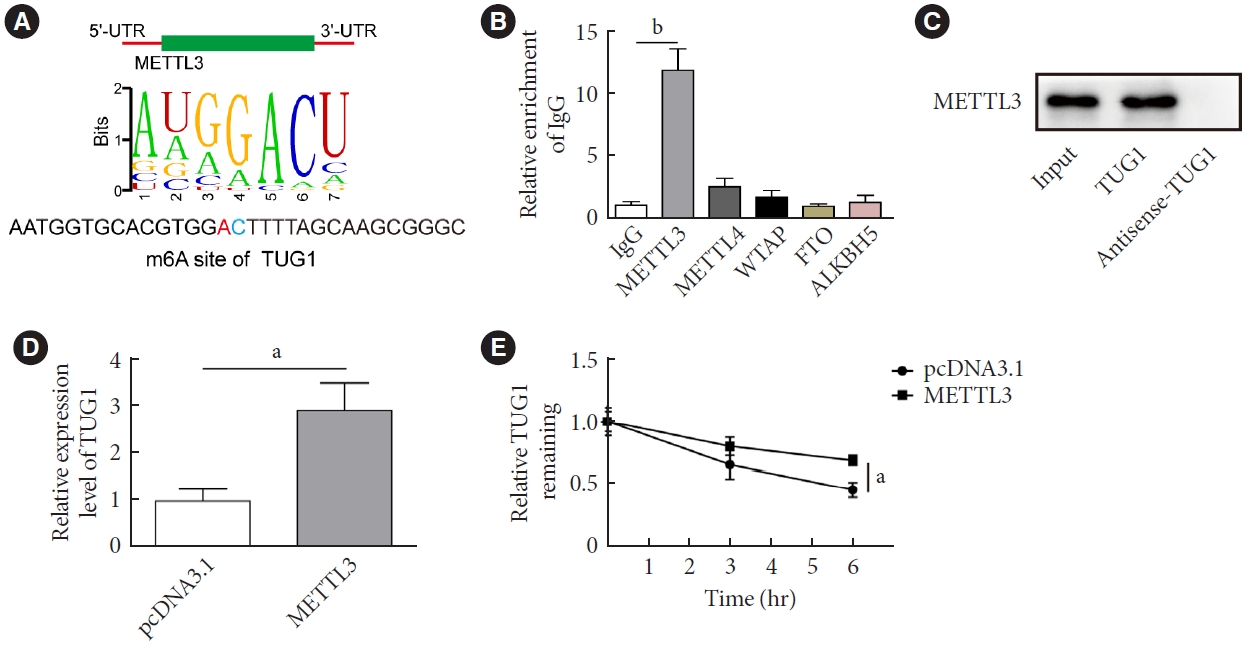
The present study investigated the regulatory effects of N6-methyladenosine (m6A) methyltransferase like-3 (METTL3) in diabetes-induced testicular damage.
Methods
In vivo diabetic mice and high glucose (HG) treated GC-1 spg cells were established. The mRNA and protein expressions were determined by real-time quantitative polymerase chain reaction, Western blot, immunofluorescence and immunohistochemistry staining. Levels of testosterone, blood glucose, cell viability, and apoptosis were detected by enzyme-linked immunosorbent assay, MTT, and flow cytometry, respectively. Molecular interactions were verified by RNA immunoprecipitation and RNA pull-down assay. Histopathological staining was performed to evaluate testicular injury.
Results
METTL3 and long non-coding RNA taurine up-regulated 1 (lncRNA TUG1) were downregulated in testicular tissues of diabetic mice and HG-treated GC-1 spg cells. METTL3 overexpression could reduce the blood glucose level, oxidative stress and testicular damage but enhance testosterone secretion in diabetic mouse model and HG-stimulated GC-1 spg cells. Mechanically, METTL3-mediated m6A methylation enhanced the stability of TUG1, then stabilizing the clusterin mRNA via recruiting serine and arginine rich splicing factor 1. Moreover, inhibition of TUG1/clusterin signaling markedly reversed the protective impacts of METTL3 overexpression on HG-stimulated GC-1 spg cells.
Conclusion
This study demonstrated that METTL3 ameliorated diabetes-induced testicular damage by upregulating the TUG1/clusterin signaling. These data further elucidate the potential regulatory mechanisms of m6A modification on diabetes-induced testicular injury. -
Citations
Citations to this article as recorded by- Negative Regulation of LINC01013 by METTL3 and YTHDF2 Enhances the Osteogenic Differentiation of Senescent Pre‐Osteoblast Cells Induced by Hydrogen Peroxide
Jiaxin Song, Yuejun Wang, Zhao Zhu, Wanqing Wang, Haoqing Yang, Zhaochen Shan
Advanced Biology.2024;[Epub] CrossRef - Diabetes and diabetic associative diseases: An overview of epigenetic regulations of TUG1
Mohammed Ageeli Hakami
Saudi Journal of Biological Sciences.2024; 31(5): 103976. CrossRef
- Negative Regulation of LINC01013 by METTL3 and YTHDF2 Enhances the Osteogenic Differentiation of Senescent Pre‐Osteoblast Cells Induced by Hydrogen Peroxide
- Basic Research
- Role of Autophagy in Granulocyte-Colony Stimulating Factor Induced Anti-Apoptotic Effects in Diabetic Cardiomyopathy
- Guang-Yin Shen, Jeong-Hun Shin, Yi-Sun Song, Hyun-Woo Joo, In-Hwa Park, Jin-Hee Seong, Na-Kyoung Shin, A-Hyeon Lee, Young Jong Cho, Yonggu Lee, Young-Hyo Lim, Hyuck Kim, Kyung-Soo Kim
- Diabetes Metab J. 2021;45(4):594-605. Published online February 26, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0049
- 7,213 View
- 147 Download
- 3 Web of Science
- 2 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
- Background
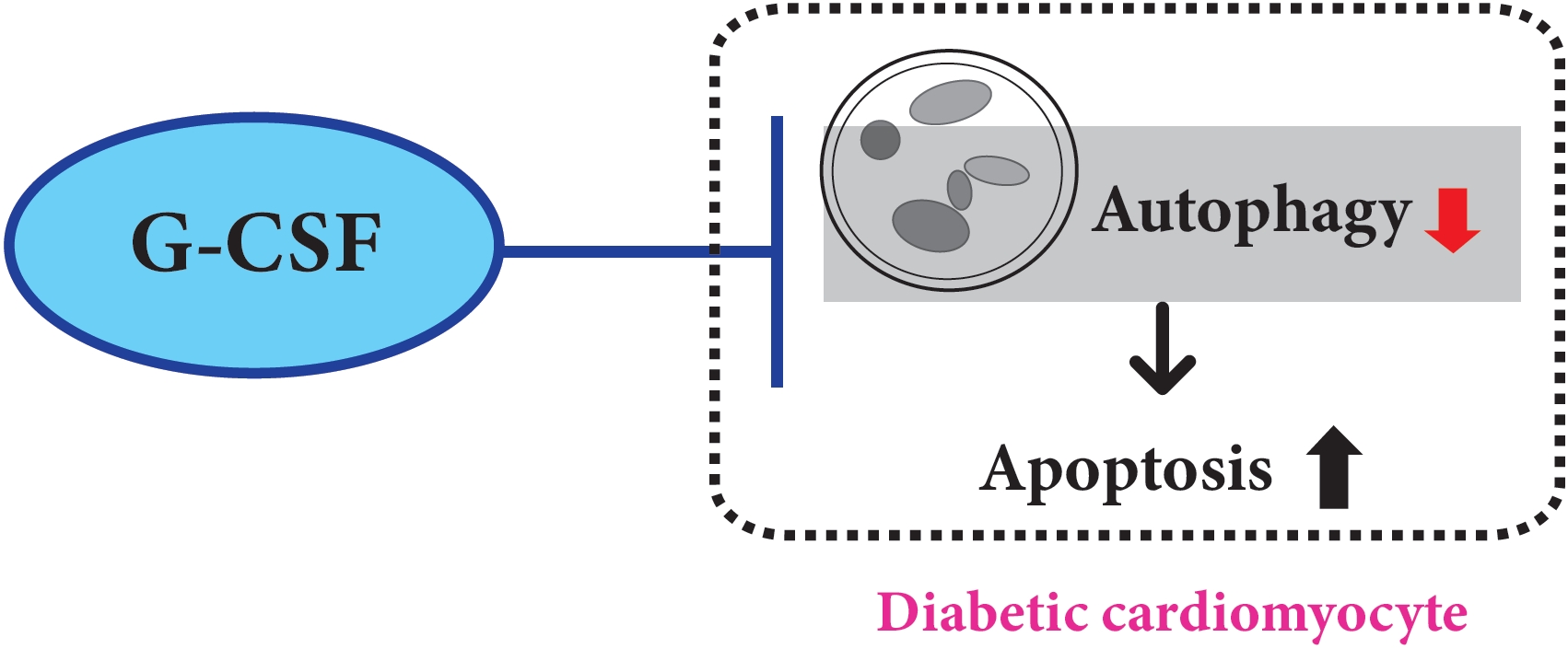
We previously, reported that granulocyte-colony stimulating factor (G-CSF) reduces cardiomyocyte apoptosis in diabetic cardiomyopathy. However, the underlying mechanisms are not yet fully understood. Therefore, we investigated whether the mechanisms underlying of the anti-apoptotic effects of G-CSF were associated with autophagy using a rat model of diabetic cardiomyopathy.
Methods
Diabetic cardiomyopathy was induced in rats through a high-fat diet combined with low-dose streptozotocin and the rats were then treated with G-CSF for 5 days. Rat H9c2 cardiac cells were cultured under high glucose conditions as an in vitro model of diabetic cardiomyopathy. The extent of apoptosis and protein levels related to autophagy (Beclin-1, microtubule-binding protein light chain 3 [LC3]-II/LC3-I ratio, and P62) were determined for both models. Autophagy determination was performed using an Autophagy Detection kit.
Results
G-CSF significantly reduced cardiomyocyte apoptosis in the diabetic myocardium in vivo and led to an increase in Beclin-1 level and the LC3-II/LC3-I ratio, and decreased P62 level. Similarly, G-CSF suppressed apoptosis, increased Beclin-1 level and LC3-II/LC3-I ratio, and decreased P62 level in high glucose-induced H9c2 cardiac cells in vitro. These effects of G-CSF were abrogated by 3-methyladenine, an autophagy inhibitor. In addition, G-CSF significantly increased autophagic flux in vitro.
Conclusion
Our results suggest that the anti-apoptotic effect of G-CSF might be significantly associated with the up-regulation of autophagy in diabetic cardiomyopathy. -
Citations
Citations to this article as recorded by- Ginkgo biloba extract protects against diabetic cardiomyopathy by restoring autophagy via adenosine monophosphate‐activated protein kinase/mammalian target of the rapamycin pathway modulation
Xueyao Yang, Xin Zhao, Yanfei Liu, Yue Liu, Libo Liu, Ziyu An, Haoran Xing, Jinfan Tian, Xiantao Song
Phytotherapy Research.2023; 37(4): 1377. CrossRef - Perspectives for Forkhead box transcription factors in diabetic cardiomyopathy: Their therapeutic potential and possible effects of salvianolic acids
Ronghui Han, Hemeng Huang, Weiyi Xia, Jingjin Liu, Hui Luo, Jing Tang, Zhengyuan Xia
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef
- Ginkgo biloba extract protects against diabetic cardiomyopathy by restoring autophagy via adenosine monophosphate‐activated protein kinase/mammalian target of the rapamycin pathway modulation
- Islet Studies and Transplantation
-
- Myricetin Protects Against High Glucose-Induced β-Cell Apoptosis by Attenuating Endoplasmic Reticulum Stress via Inactivation of Cyclin-Dependent Kinase 5
- Udayakumar Karunakaran, Suma Elumalai, Jun Sung Moon, Jae-Han Jeon, Nam Doo Kim, Keun-Gyu Park, Kyu Chang Won, Jaechan Leem, In-Kyu Lee
- Diabetes Metab J. 2019;43(2):192-205. Published online January 16, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0052
- 4,903 View
- 106 Download
- 33 Web of Science
- 32 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background Chronic hyperglycemia has deleterious effects on pancreatic β-cell function and turnover. Recent studies support the view that cyclin-dependent kinase 5 (CDK5) plays a role in β-cell failure under hyperglycemic conditions. However, little is known about how CDK5 impair β-cell function. Myricetin, a natural flavonoid, has therapeutic potential for the treatment of type 2 diabetes mellitus. In this study, we examined the effect of myricetin on high glucose (HG)-induced β-cell apoptosis and explored the relationship between myricetin and CDK5.
Methods To address this question, we subjected INS-1 cells and isolated rat islets to HG conditions (30 mM) in the presence or absence of myricetin. Docking studies were conducted to validate the interaction between myricetin and CDK5. Gene expression and protein levels of endoplasmic reticulum (ER) stress markers were measured by real-time reverse transcription polymerase chain reaction and Western blot analysis.
Results Activation of CDK5 in response to HG coupled with the induction of ER stress via the down regulation of sarcoendoplasmic reticulum calcium ATPase 2b (
SERCA2b ) gene expression and reduced the nuclear accumulation of pancreatic duodenal homeobox 1 (PDX1) leads to β-cell apoptosis. Docking study predicts that myricetin inhibit CDK5 activation by direct binding in the ATP-binding pocket. Myricetin counteracted the decrease in the levels of PDX1 and SERCA2b by HG. Moreover, myricetin attenuated HG-induced apoptosis in INS-1 cells and rat islets and reduce the mitochondrial dysfunction by decreasing reactive oxygen species production and mitochondrial membrane potential (Δψm) loss.Conclusion Myricetin protects the β-cells against HG-induced apoptosis by inhibiting ER stress, possibly through inactivation of CDK5 and consequent upregulation of PDX1 and SERCA2b.
-
Citations
Citations to this article as recorded by- Profiling of secondary metabolite and evaluation of anti-diabetic potency of Crotalaria quinquefolia (L): In-vitro, in-vivo, and in-silico approaches
Nazmun Nahar, Md. Nazmul Hasan Zilani, Partha Biswas, Md. Morsaline Billah, Shabana Bibi, Norah A. Albekairi, Abdulrahman Alshammari, Md. Nazmul Hasan
Saudi Pharmaceutical Journal.2024; 32(1): 101887. CrossRef - Mitochondrial aldehyde dehydrogenase-2 coordinates the hydrogen sulfide - AMPK axis to attenuate high glucose-induced pancreatic β-cell dysfunction by glutathione antioxidant system
Udayakumar Karunakaran, Suma Elumalai, Seung Min Chung, Kathrin Maedler, Kyu Chang Won, Jun Sung Moon
Redox Biology.2024; 69: 102994. CrossRef - Network-based identification and mechanism exploration of active ingredients against Alzheimer’s disease via targeting endoplasmic reticulum stress from traditional chinese medicine
Zhao Dai, Tian Hu, Junwen Wei, Xue Wang, Chuipu Cai, Yong Gu, Yunhui Hu, Wenjia Wang, Qihui Wu, Jiansong Fang
Computational and Structural Biotechnology Journal.2024; 23: 506. CrossRef - Myricetin as a Promising Flavonoid with Multitargeted Biological Activity
A.S. Chiriapkin
Juvenis Scientia.2024; 10(1): 5. CrossRef - Naturally occurring small molecules with dual effect upon inflammatory signaling pathways and endoplasmic reticulum stress response
Daniela Correia da Silva, Patrícia Valentão, David M. Pereira
Journal of Physiology and Biochemistry.2024;[Epub] CrossRef - Omnifarious fruit polyphenols: an omnipotent strategy to prevent and intervene diabetes and related complication?
Yao Chen, Xuejiao Qie, Wei Quan, Maomao Zeng, Fang Qin, Jie Chen, Benu Adhikari, Zhiyong He
Critical Reviews in Food Science and Nutrition.2023; 63(20): 4288. CrossRef - Regulation of reactive oxygen species by phytochemicals for the management of cancer and diabetes
Heui Min Lim, See-Hyoung Park
Critical Reviews in Food Science and Nutrition.2023; 63(22): 5911. CrossRef - Bioactive compounds from Polygonatum genus as anti-diabetic agents with future perspectives
Yan Shi, Dun Si, Donghong Chen, Xinfeng Zhang, Zhigang Han, Qiang Yu, Jingjing Liu, Jinping Si
Food Chemistry.2023; 408: 135183. CrossRef - Venom Peptides, Polyphenols and Alkaloids: Are They the Next Antidiabetics That Will Preserve β-Cell Mass and Function in Type 2 Diabetes?
Michele Lodato, Valérie Plaisance, Valérie Pawlowski, Maxime Kwapich, Alexandre Barras, Emeline Buissart, Stéphane Dalle, Sabine Szunerits, Jérôme Vicogne, Rabah Boukherroub, Amar Abderrahmani
Cells.2023; 12(6): 940. CrossRef - TFP5 attenuates cyclin‐dependent kinase 5‐mediated islet β‐cell damage in diabetes
Shunyao Liu, Bo Li, Danna Ma, Yuejia Tao, Jiang Song, Li Bao, Guoqing Zhang, Hongyan Luo, Shilu Cao, Jing E, Yali Zheng
Chemical Biology & Drug Design.2023; 102(1): 76. CrossRef - Antiviral and Possible Prophylactic Significance of Myricetin for COVID-19
Pawan K. Agrawal, Chandan Agrawal, Gerald Blunden
Natural Product Communications.2023; 18(4): 1934578X2311662. CrossRef - In Vitro and In Silico Protocols for the Assessment of Anti-Tick Compounds from Pinus roxburghii against Rhipicephalus (Boophilus) microplus Ticks
Sana Ayub, Nosheen Malak, Raquel Cossío-Bayúgar, Nasreen Nasreen, Afshan Khan, Sadaf Niaz, Adil Khan, Abdallah D. Alanazi, Mourad Ben Said
Animals.2023; 13(8): 1388. CrossRef - Protective Effect of Myricetin Against Experimentally Induced Torsion in Rats
M. Tatar, Z. Polat, J. Öner, H. Öner
Biology Bulletin.2023; 50(6): 1338. CrossRef - The pharmacological mechanism of Abelmoschus manihot in the treatment of chronic kidney disease
Cuiting Wei, Chao Wang, Run Li, Yunfeng Bai, Xue Wang, Qingyun Fang, Xiangmei Chen, Ping Li
Heliyon.2023; 9(11): e22017. CrossRef - Natural Polyphenols as SERCA Activators: Role in the Endoplasmic Reticulum Stress-Related Diseases
Jana Viskupicova, Petronela Rezbarikova
Molecules.2022; 27(16): 5095. CrossRef - Research progress on the mechanism of beta-cell apoptosis in type 2 diabetes mellitus
SuFang You, JingYi Zheng, YuPing Chen, HuiBin Huang
Frontiers in Endocrinology.2022;[Epub] CrossRef - Myricetin inhibits pseudorabies virus infection through direct inactivation and activating host antiviral defense
Huaiyue Hu, Zhiqiang Hu, Yingying Zhang, Hongping Wan, Zhongqiong Yin, Lixia Li, Xiaoxia Liang, Xinghong Zhao, Lizi Yin, Gang Ye, Yuan-Feng Zou, Huaqiao Tang, Renyong Jia, Yaqin Chen, Hao Zhou, Xu Song
Frontiers in Microbiology.2022;[Epub] CrossRef - Effects of myricetin against cadmium-induced neurotoxicity in PC12 cells
Azadeh Aminzadeh, Ayda Salarinejad
Toxicology Research.2021; 10(1): 84. CrossRef - Pioglitazone-induced AMPK-Glutaminase-1 prevents high glucose-induced pancreatic β-cell dysfunction by glutathione antioxidant system
Udayakumar Karunakaran, Suma Elumalai, Jun Sung Moon, Kyu Chang Won
Redox Biology.2021; 45: 102029. CrossRef - Chlorogenic acid and β-glucan from highland barley grain ameliorate β-cell dysfunction via inhibiting apoptosis and improving cell proliferation
Ze-Hua Liu, Bo Li
Food & Function.2021; 12(20): 10040. CrossRef - The cyclin dependent kinase inhibitor Roscovitine prevents diet-induced metabolic disruption in obese mice
Nabil Rabhi, Kathleen Desevin, Briana Noel Cortez, Ryan Hekman, Jean Z. Lin, Andrew Emili, Stephen R. Farmer
Scientific Reports.2021;[Epub] CrossRef - AdipoRon promotes diabetic fracture repair through endochondral ossification-based bone repair by enhancing survival and differentiation of chondrocytes
Zhongyi Wang, Jinxin Tang, Ying Li, Yu Wang, Yanyang Guo, Qisheng Tu, Jake Chen, Chen Wang
Experimental Cell Research.2020; 387(2): 111757. CrossRef - A kinase of many talents: non-neuronal functions of CDK5 in development and disease
Samanta Sharma, Piotr Sicinski
Open Biology.2020; 10(1): 190287. CrossRef - Mitochondrial dysfunction in the fetoplacental unit in gestational diabetes mellitus
Luis Sobrevia, Paola Valero, Adriana Grismaldo, Roberto Villalobos-Labra, Fabián Pardo, Mario Subiabre, Gael Armstrong, Fernando Toledo, Sofía Vega, Marcelo Cornejo, Gonzalo Fuentes, Reinaldo Marín
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2020; 1866(12): 165948. CrossRef - Myricetin bioactive effects: moving from preclinical evidence to potential clinical applications
Yasaman Taheri, Hafiz Ansar Rasul Suleria, Natália Martins, Oksana Sytar, Ahmet Beyatli, Balakyz Yeskaliyeva, Gulnaz Seitimova, Bahare Salehi, Prabhakar Semwal, Sakshi Painuli, Anuj Kumar, Elena Azzini, Miquel Martorell, William N. Setzer, Alfred Maroyi,
BMC Complementary Medicine and Therapies.2020;[Epub] CrossRef - Current Pharmacological Trends on Myricetin
Gudiya Gupta, Mohd Aftab Siddiqui, Mohd Muazzam Khan, Mohd Ajmal, Rabiya Ahsan, Md Azizur Rahaman, Md Afroz Ahmad, Md Arshad, Mohammad Khushtar
Drug Research.2020;[Epub] CrossRef - Silencing cyclophilin A improves insulin secretion, reduces cell apoptosis, and alleviates inflammation as well as oxidant stress in high glucose-induced pancreatic β-cells via MAPK/NF-kb signaling pathway
Tangying Li, Huibiao Quan, Huachuan Zhang, Leweihua Lin, Qianying Ou, Kaining Chen
Bioengineered.2020; 11(1): 1047. CrossRef - Endoplasmic reticulum stress contributes to NMDA-induced pancreatic β-cell dysfunction in a CHOP-dependent manner
Xiao-Ting Huang, Wei Liu, Yong Zhou, Mei Sun, Chen-Chen Sun, Chen-Yu Zhang, Si-Yuan Tang
Life Sciences.2019; 232: 116612. CrossRef - Dapagliflozin rescues endoplasmic reticulum stress-mediated cell death
Ryo Shibusawa, Eijiro Yamada, Shuichi Okada, Yasuyo Nakajima, Claire C. Bastie, Akito Maeshima, Kyoichi Kaira, Masanobu Yamada
Scientific Reports.2019;[Epub] CrossRef - Myricetin prevents thapsigargin-induced CDK5-P66Shc signalosome mediated pancreatic β-cell dysfunction
Udayakumar Karunakaran, Ji Eun Lee, Suma Elumalai, Jun Sung Moon, Kyu Chang Won
Free Radical Biology and Medicine.2019; 141: 59. CrossRef - CDK5: Key Regulator of Apoptosis and Cell Survival
Rabih Roufayel, Nimer Murshid
Biomedicines.2019; 7(4): 88. CrossRef - Oral DhHP-6 for the Treatment of Type 2 Diabetes Mellitus
Kai Wang, Yu Su, Yuting Liang, Yanhui Song, Liping Wang
International Journal of Molecular Sciences.2019; 20(6): 1517. CrossRef
- Profiling of secondary metabolite and evaluation of anti-diabetic potency of Crotalaria quinquefolia (L): In-vitro, in-vivo, and in-silico approaches
- Others
- Repeated Glucose Deprivation/Reperfusion Induced PC-12 Cell Death through the Involvement of FOXO Transcription Factor
- Na Han, You Jeong Kim, Su Min Park, Seung Man Kim, Ji Suk Lee, Hye Sook Jung, Eun Ju Lee, Tae Kyoon Kim, Tae Nyun Kim, Min Jeong Kwon, Soon Hee Lee, Mi-kyung Kim, Byoung Doo Rhee, Jeong Hyun Park
- Diabetes Metab J. 2016;40(5):396-405. Published online September 1, 2016
- DOI: https://doi.org/10.4093/dmj.2016.40.5.396
- 4,669 View
- 30 Download
- 2 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Cognitive impairment and brain damage in diabetes is suggested to be associated with hypoglycemia. The mechanisms of hypoglycemia-induced neural death and apoptosis are not clear and reperfusion injury may be involved. Recent studies show that glucose deprivation/reperfusion induced more neuronal cell death than glucose deprivation itself. The forkhead box O (FOXO) transcription factors are implicated in the regulation of cell apoptosis and survival, but their role in neuronal cells remains unclear. We examined the role of FOXO transcription factors and the involvement of the phosphatidylinositol 3-kinase (PI3K)/Akt and apoptosis-related signaling pathways in PC-12 cells exposed to repeated glucose deprivation/reperfusion.
Methods PC-12 cells were exposed to control (Dulbecco's Modified Eagle Medium [DMEM] containing 25 mM glucose) or glucose deprivation/reperfusion (DMEM with 0 mM glucose for 6 hours and then DMEM with 25 mM glucose for 18 hours) for 5 days. MTT assay and Western blot analysis were performed for cell viability, apoptosis, and the expression of survival signaling pathways. FOXO3/4',6-diamidino-2-phenylindole staining was done to ascertain the involvement of FOXO transcription factors in glucose deprivation/reperfusion conditions.
Results Compared to PC-12 cells not exposed to hypoglycemia, cells exposed to glucose deprivation/reperfusion showed a reduction of cell viability, decreased expression of phosphorylated Akt and Bcl-2, and an increase of cleaved caspase-3 expression. Of note, FOXO3 protein was localized in the nuclei of glucose deprivation/reperfusion cells but not in the control cells.
Conclusion Repeated glucose deprivation/reperfusion caused the neuronal cell death. Activated FOXO3 via the PI3K/Akt pathway in repeated glucose deprivation/reperfusion was involved in genes related to apoptosis.
-
Citations
Citations to this article as recorded by- Predictive factors for the development of diabetes in cancer patients treated with phosphatidylinositol 3-kinase inhibitors
Gyuri Kim, Myungeun Yoo, Min Hee Hong, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Hye Ryun Kim, Yong-ho Lee, Byoung Chul Cho
Cancer Chemotherapy and Pharmacology.2019; 84(2): 405. CrossRef
- Predictive factors for the development of diabetes in cancer patients treated with phosphatidylinositol 3-kinase inhibitors
- Others
- Metformin Promotes Apoptosis but Suppresses Autophagy in Glucose-Deprived H4IIE Hepatocellular Carcinoma Cells
- Deok-Bae Park
- Diabetes Metab J. 2015;39(6):518-527. Published online December 11, 2015
- DOI: https://doi.org/10.4093/dmj.2015.39.6.518
- 4,114 View
- 42 Download
- 16 Web of Science
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Metformin, a well-known anti-diabetic drug, has gained interest due to its association with the reduction of the prevalence of cancer in patients with type 2 diabetes and the anti-proliferative effect of metformin in several cancer cells. Here, we investigated the anti-proliferative effect of metformin with respect to apoptosis and autophagy in H4IIE hepatocellular carcinoma cells.
Methods H4IIE rat cells were treated with metformin in glucose-free medium for 24 hours and were then subjected to experiments examining the onset of apoptosis and/or autophagy as well as the related signaling pathways.
Results When H4IIE cells were incubated in glucose-free media for 24 hours, metformin and 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) reduced the viability of cells. Inhibition of AMP-activated protein kinase (AMPK) by compound C significantly blocked cell death induced by metformin or AICAR. Pro-apoptotic events (nuclear condensation, hydrolysis of intact poly ADP ribose polymerase and caspase-3) were stimulated by metformin and then suppressed by compound C. Interestingly, the formation of acidic intracellular vesicles, a marker of autophagy, was stimulated by compound C. Although the deprivation of amino acids in culture media also induced apoptosis, neither metformin nor compound C affected cell viability. The expression levels of all of the autophagy-related proteins examined decreased with metformin, and two proteins (light chain 3 and beclin-1) were sensitive to compound C. Among the tested inhibitors against MAP kinases and phosphatidylinositol-3-kinase/mammalian target of rapamycin, SB202190 (against p38MAP kinase) significantly interrupted the effects of metformin.
Conclusion Our data suggest that metformin induces apoptosis, but suppresses autophagy, in hepatocellular carcinoma cells via signaling pathways, including AMPK and p38 mitogen-activated protein kinase.
-
Citations
Citations to this article as recorded by- Metformin Induces Lipogenesis and Apoptosis in H4IIE Hepatocellular
Carcinoma Cells
Deokbae Park, Sookyoung Lee, Hyejin Boo
Development & Reproduction.2023; 27(2): 77. CrossRef - Novel phloretin-based combinations targeting glucose metabolism in hepatocellular carcinoma through GLUT2/PEPCK axis of action: in silico molecular modelling and in vivo studies
Alaa Elmetwalli, Neamat H. Kamosh, Rania El Safty, Amany I. Youssef, Mohammed M. Salama, Khaled M. Abd El-Razek, Tarek El-Sewedy
Medical Oncology.2023;[Epub] CrossRef - Targeted Pyroptosis Is a Potential Therapeutic Strategy for Cancer
Hao Wu, Dianlun Qian, Xiangfeng Bai, Shibo Sun, Jayaprakash Narayana Kolla
Journal of Oncology.2022; 2022: 1. CrossRef - The effects of metformin on autophagy
Guangli Lu, Zhen Wu, Jia Shang, Zhenxing Xie, Chaoran Chen, Chuning zhang
Biomedicine & Pharmacotherapy.2021; 137: 111286. CrossRef - Protective Effect of Metformin against Hydrogen Peroxide-Induced Oxidative Damage in Human Retinal Pigment Epithelial (RPE) Cells by Enhancing Autophagy through Activation of AMPK Pathway
Xia Zhao, Linlin Liu, Yizhou Jiang, Marta Silva, Xuechu Zhen, Wenhua Zheng
Oxidative Medicine and Cellular Longevity.2020; 2020: 1. CrossRef Metformin Induces Autophagy via the AMPK-mTOR Signaling Pathway in Human Hepatocellular Carcinoma Cells
Chun Gao, Long Fang, Hui Zhang, Wei-Shuo Zhang, Xiao-Ou Li, Shi-Yu Du
Cancer Management and Research.2020; Volume 12: 5803. CrossRef- Metabolomics profiling of metformin-mediated metabolic reprogramming bypassing AMPKα
Min Yan, Huan Qi, Tian Xia, Xinjie Zhao, Wen Wang, Zhichao Wang, Chang Lu, Zhen Ning, Huan Chen, Tongming Li, Dinesh Singh Tekcham, Xiumei Liu, Jing Liu, Di Chen, Xiaolong Liu, Guowang Xu, Hai-long Piao
Metabolism.2019; 91: 18. CrossRef - Metformin Induces Oxidative Stress-Mediated Apoptosis without the Blockade of Glycolysis in H4IIE Hepatocellular Carcinoma Cells
Deokbae Park
Biological and Pharmaceutical Bulletin.2019; 42(12): 2002. CrossRef - Activation of AMPK prevents monocrotaline-induced pulmonary arterial hypertension by suppression of NF-κB-mediated autophagy activation
Cui Zhai, Wenhua Shi, Wei Feng, Yanting Zhu, Jian Wang, Shaojun Li, Xin Yan, Qingting Wang, Qianqian Zhang, Limin Chai, Cong Li, Pengtao Liu, Manxiang Li
Life Sciences.2018; 208: 87. CrossRef - Metformin and epothilone A treatment up regulate pro-apoptotic PARP-1, Casp-3 and H2AX genes and decrease of AKT kinase level to control cell death of human hepatocellular carcinoma and ovary adenocarcinoma cells
Aneta Rogalska, Barbara Bukowska, Agnieszka Marczak
Toxicology in Vitro.2018; 47: 48. CrossRef - Quantitative assessment of cell fate decision between autophagy and apoptosis
Bing Liu, Zoltán N. Oltvai, Hülya Bayır, Gary A. Silverman, Stephen C. Pak, David H. Perlmutter, Ivet Bahar
Scientific Reports.2017;[Epub] CrossRef - Meta-analysis of studies using metformin as a reducer for liver cancer risk in diabetic patients
Shujuan Ma, Yixiang Zheng, Yanni Xiao, Pengcheng Zhou, Hongzhuan Tan
Medicine.2017; 96(19): e6888. CrossRef - ROS Production and ERK Activity Are Involved in the Effects of d-β-Hydroxybutyrate and Metformin in a Glucose Deficient Condition
Santosh Lamichhane, Tonking Bastola, Ramesh Pariyar, Eun-Sol Lee, Ho-Sub Lee, Dae Lee, Jungwon Seo
International Journal of Molecular Sciences.2017; 18(3): 674. CrossRef - Metformin represses glucose starvation induced autophagic response in microvascular endothelial cells and promotes cell death
Samson Mathews Samuel, Suparna Ghosh, Yasser Majeed, Gnanapragasam Arunachalam, Mohamed M. Emara, Hong Ding, Chris R. Triggle
Biochemical Pharmacology.2017; 132: 118. CrossRef - NHX-5, an Endosomal Na+/H+ Exchanger, Is Associated with Metformin Action
Jeongho Kim, Hye-Yeon Lee, Jheesoo Ahn, Moonjung Hyun, Inhwan Lee, Kyung-Jin Min, Young-Jai You
Journal of Biological Chemistry.2016; 291(35): 18591. CrossRef - Metformin in pancreatic cancer treatment: from clinical trials through basic research to biomarker quantification
Archana Bhaw-Luximon, Dhanjay Jhurry
Journal of Cancer Research and Clinical Oncology.2016; 142(10): 2159. CrossRef - Metformina: stary lek w nowej aplikacji
Anna Dmoszyńska, Monika Podhorecka, Krzysztof Giannopoulos
Acta Haematologica Polonica.2016; 47(2): 139. CrossRef
- Metformin Induces Lipogenesis and Apoptosis in H4IIE Hepatocellular
Carcinoma Cells
- The Effects of Glyburide on Apoptosis and Endoplasmic Reticulum Stress in INS-1 Cells in a Glucolipotoxic Condition
- Min Jeong Kwon, Hye Suk Chung, Chang Shin Yoon, Jung Hae Ko, Hae Jung Jun, Tae Kyun Kim, Soon Hee Lee, Kyung Soo Ko, Byoung Doo Rhee, Mi Kyung Kim, Jeong Hyun Park
- Diabetes Metab J. 2011;35(5):480-488. Published online October 31, 2011
- DOI: https://doi.org/10.4093/dmj.2011.35.5.480
- 3,912 View
- 43 Download
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background β-cell death due to endoplasmic reticulum (ER) stress has been regarded as an important pathogenic component of type 2 diabetes. The possibility has been suggested that sulfonylurea, currently being used as one of the main oral hypoglycemic agents of type 2 diabetes, increases ER stress, which could lead to sulfonylurea failure. The authors of the present study examined ER stress of β-cells in a glucolipotoxic condition using glyburide (GB) in an environment mimicking type 2 diabetes.
Methods Apoptosis was induced by adding various concentrations of GB (0.001 to 200 µM) to a glucolipotoxic condition using 33 mM glucose, and the effects of varied concentrations of palmitate were evaluated via annexin V staining. The markers of ER stress and pro-apoptotic markers were assessed by Western blotting and semi-quantitative reverse transcription-polymerase chain reaction. Additionally, the anti-apoptotic markers were evaluated.
Results Addition of any concentration of GB in 150 µM palmitate and 33 mM glucose did not increase apoptosis. The expression of phosphorylated eukaryotic initiation factor (eIF-2α) was increased and cleaved caspase 3 was decreased by adding GB to a glucolipotoxic condition. However, other ER stress-associated markers such as Bip-1, X-box binding protein-1, ATF-4 and C/EBP-homologous protein transcription factor and anti-apoptotic markers phosphor-p85 phosphatidylinositol 3-kinase and phosphorylation of Akt did not change significantly.
Conclusion GB did not show further deleterious effects on the degree of apoptosis or ER stress of INS-1 cells in a glucolipotoxic condition. Increased phosphorylation of eIF-2α may attenuate ER stress for adaptation to increased ER protein load.
-
Citations
Citations to this article as recorded by- The antagonistic atorvastatin-glibenclamide interactions suppressed the atorvastatin-induced Bax/cytochrome c/p53 mRNA expressions and increased Rho A mRNA expression in B16f10 melanoma cell culture
Maryam Malek, Nasim Dana, Ahmad Ghasemi, Maedeh Ghasemi
Gene Reports.2021; 23: 101156. CrossRef - Expression profiles of stress-related genes in islets from donors with progressively impaired glucose metabolism
Marcus Lundberg, Anton Stenwall, Angie Tegehall, Olle Korsgren, Oskar Skog
Islets.2018; 10(2): 69. CrossRef - Pharmacological Modulators of Endoplasmic Reticulum Stress in Metabolic Diseases
Tae Jung, Kyung Choi
International Journal of Molecular Sciences.2016; 17(2): 192. CrossRef - The TRPA1 channel and oral hypoglycemic agents
Carlos Manlio Diaz-Garcia
Channels.2013; 7(6): 420. CrossRef - Dimethyl sulfoxide reduces hepatocellular lipid accumulation through autophagy induction
Young Mi Song, Sun-Ok Song, Yong-Keun Jung, Eun-Seok Kang, Bong Soo Cha, Hyun Chul Lee, Byung-Wan Lee
Autophagy.2012; 8(7): 1085. CrossRef - The Duration of Sulfonylurea Treatment Is Associated withβ-Cell Dysfunction in Patients with Type 2 Diabetes Mellitus
Mi-Seon Shin, Jee Hee Yu, Chang Hee Jung, Jenie Yoonoo Hwang, Woo Je Lee, Min-Seon Kim, Joong-Yeol Park
Diabetes Technology & Therapeutics.2012; 14(11): 1033. CrossRef
- The antagonistic atorvastatin-glibenclamide interactions suppressed the atorvastatin-induced Bax/cytochrome c/p53 mRNA expressions and increased Rho A mRNA expression in B16f10 melanoma cell culture
- The Effect of Glucose Fluctuation on Apoptosis and Function of INS-1 Pancreatic Beta Cells
- Mi Kyung Kim, Hye Sook Jung, Chang Shin Yoon, Jung Hae Ko, Hae Jung Jun, Tae Kyun Kim, Min Jeong Kwon, Soon Hee Lee, Kyung Soo Ko, Byoung Doo Rhee, Jeong Hyun Park
- Korean Diabetes J. 2010;34(1):47-54. Published online February 28, 2010
- DOI: https://doi.org/10.4093/kdj.2010.34.1.47
- 3,803 View
- 30 Download
- 18 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Blood glucose level continuously fluctuates within a certain range in the human body. In diabetes patients, the extent of such fluctuation is large, despite the strict control of blood glucose. Blood glucose fluctuation has been shown to mediate more adverse effects on vascular endothelial cells and diabetes complications than chronic hyperglycemia, which has been explained as due to oxidative stress. As few previous studies have reported the effects of chronic and intermittent hyperglycemia on the apoptosis and function of pancreatic beta cells, this study reported herein was performed to investigate such effects on these cells.
Methods For chronic hyperglycemia, INS-1 cells were cultured for 5 days with changes of RPMI 1640 medium containing 33 mM glucose every 12 hours. For intermittent hyperglycemia, the medium containing 11 mM glucose was exchanged with the medium containing 33 mM glucose every 12 hours. Apoptosis was assessed by TUNEL assay Hoechst staining and cleaved caspase 3. Insulin secretory capacity was assessed, and the expression of Mn-SOD and Bcl-2 was measured by Western blotting.
Results In comparison to the control group, INS-1 cells exposed to chronic hyperglycemia and intermittent hyperglycemia showed an increase in apoptosis. The apoptosis of INS-1 cells exposed to intermittent hyperglycemia increased significantly more than the apoptosis of INS-1 cells exposed to chronic hyperglycemia. In comparison to the control group, the insulin secretory capacity in the two hyperglycemic states was decreased, and more with intermittent hyperglycemia than with chronic hyperglycemia. The expression of Mn-SOD and Bcl-2 increased more with chronic hyperglycemia than with intermittent hyperglycemia.
Conclusion Intermittent hyperglycemia induced a higher degree of apoptosis and decreased the insulin secretory capacity more in pancreatic beta cells than chronic hyperglycemia. This activity may be mediated by the anti-oxidative enzyme Mn-SOD and the anti-apoptotic signal Bcl-2.
-
Citations
Citations to this article as recorded by- Association between hemoglobin glycation index and diabetic kidney disease in type 2 diabetes mellitus in China: A cross- sectional inpatient study
Sixu Xin, Xin Zhao, Jiaxiang Ding, Xiaomei Zhang
Frontiers in Endocrinology.2023;[Epub] CrossRef - Plant polyphenols mechanisms of action on insulin resistance and against the loss of pancreatic beta cells
Camelia Papuc, Gheorghe V. Goran, Corina N. Predescu, Liliana Tudoreanu, Georgeta Ștefan
Critical Reviews in Food Science and Nutrition.2022; 62(2): 325. CrossRef - Correlation between HbA1c and Triglyceride Level with Coronary Stenosis Degree in Type 2 Diabetes Mellitus with Coronary Heart Disease
Laily Adninta, Indranila Samsuria, Edward Kurnia Setiawan Limijadi
Open Access Macedonian Journal of Medical Sciences.2022; 10(B): 944. CrossRef - Age‐specific associations of glycated haemoglobin variability with cardiovascular disease and mortality in patients with type 2 diabetes mellitus: A 10‐ year cohort study
Eric Yuk Fai Wan, Esther Yee Tak Yu, Weng Yee Chin, Florence Ting Yan Ng, Shu Ming Cheryl Chia, Ian Chi Kei Wong, Esther Wai Yin Chan, Cindy Lo Kuen Lam
Diabetes, Obesity and Metabolism.2020; 22(8): 1316. CrossRef - Molecular Mechanisms of Glucose Fluctuations on Diabetic Complications
Zhen-Ye Zhang, Ling-Feng Miao, Ling-Ling Qian, Ning Wang, Miao-Miao Qi, Yu-Min Zhang, Shi-Peng Dang, Ying Wu, Ru-Xing Wang
Frontiers in Endocrinology.2019;[Epub] CrossRef - Resolution on the results of the first working meeting of the scientific advisory board «Actual problems of glycemic variability as a new criterion of glycemic control and safety of diabetes therapy»
Mikhail B. Antsiferov, Gagik R. Galstyan, Alexey V. Zilov, Alexander Y. Mayorov, Tatyana N. Markova, Nikolay A. Demidov, Olga M. Koteshkova, Dmitry N. Laptev, Alisa V. Vitebskaya
Diabetes mellitus.2019; 22(3): 281. CrossRef - Intermittent High Glucose Enhances the Proliferation of Rat Aortic Vascular Smooth Muscle Cells More Than Constant High Glucose via the Mitogen-Activated Protein Kinase Pathway
Sung Hoon Yu, Hyung Joon Yoo, Dong Hyun Kang, Shin Je Moon, Jae Myung Yu
Annals of Geriatric Medicine and Research.2017; 21(3): 131. CrossRef - Association of variability in hemoglobin A1c with cardiovascular diseases and mortality in Chinese patients with type 2 diabetes mellitus — A retrospective population-based cohort study
Eric Yuk Fai Wan, Colman Siu Cheung Fung, Daniel Yee Tak Fong, Cindy Lo Kuen Lam
Journal of Diabetes and its Complications.2016; 30(7): 1240. CrossRef - Ginsenoside Rg3 prevents INS-1 cell death from intermittent high glucose stress
You Jeong Kim, Su Min Park, Hye Sook Jung, Eun Ju Lee, Tae Kyoon Kim, Tae-Nyun Kim, Min Jeong Kwon, Soon Hee Lee, Byoung Doo Rhee, Mi-kyung Kim, Jeong Hyun Park
Islets.2016; 8(3): 57. CrossRef - Different antihyperglycaemic drug effects on glycaemic variability in Type 2 diabetic patients
Alina Babenko, Elena Ivanovna Krasilnikova, Nikolay Pavlovich Likhonosov, Anna Pavlovna Likhonosova, Elena Nikolaevna Grineva
Diabetes mellitus.2014; 17(4): 72. CrossRef - Exercising for Metabolic Control: Is Timing Important
Jonida Haxhi, Alessandro Scotto di Palumbo, Massimo Sacchetti
Annals of Nutrition and Metabolism.2013; 62(1): 14. CrossRef - Combined contributions of over-secreted glucagon-like peptide 1 and suppressed insulin secretion to hyperglycemia induced by gatifloxacin in rats
Yunli Yu, Xinting Wang, Can Liu, Dan Yao, Mengyue Hu, Jia Li, Nan Hu, Li Liu, Xiaodong Liu
Toxicology and Applied Pharmacology.2013; 266(3): 375. CrossRef - Blood glucose fluctuation affects skin collagen metabolism in the diabetic mouse by inhibiting the mitogen-activated protein kinase and Smad pathways
X. Ye, X. Cheng, L. Liu, D. Zhao, Y. Dang
Clinical and Experimental Dermatology.2013; 38(5): 530. CrossRef - Glucose exposure pattern determines glucagon-like peptide 1 receptor expression and signaling through endoplasmic reticulum stress in rat insulinoma cells
Ye-Hwang Cheong, Mi-Kyung Kim, Moon-Ho Son, Bong-Kiun Kaang
Biochemical and Biophysical Research Communications.2011; 414(1): 220. CrossRef - Overexpression of Insig-1 protects β cell against glucolipotoxicity via SREBP-1c
Ke Chen, ping jin, Hong-hui He, Yan-hong Xie, Xiao-yun Xie, Zhao-hui Mo
Journal of Biomedical Science.2011;[Epub] CrossRef - Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes
Gong Su, Shuhua Mi, Hong Tao, Zhao Li, Hongxia Yang, Hong Zheng, Yun Zhou, Changsheng Ma
Cardiovascular Diabetology.2011;[Epub] CrossRef - Lithospermic acid B protects beta-cells from cytokine-induced apoptosis by alleviating apoptotic pathways and activating anti-apoptotic pathways of Nrf2–HO-1 and Sirt1
Byung-Wan Lee, Sung Wan Chun, Soo Hyun Kim, Yongho Lee, Eun Seok Kang, Bong-Soo Cha, Hyun Chul Lee
Toxicology and Applied Pharmacology.2011; 252(1): 47. CrossRef - WITHDRAWN: Effect of blood glucose fluctuation on the function of rat pancreatic islets in vivo
Wang Yanjun, Xiao Yue, Li Shixing
Regulatory Peptides.2011;[Epub] CrossRef
- Association between hemoglobin glycation index and diabetic kidney disease in type 2 diabetes mellitus in China: A cross- sectional inpatient study
- Protective Effects of Glucagon Like Peptide-1 on HIT-T15 beta Cell Apoptosis via ER Stress Induced by 2-deoxy-D-glucose.
- Ju Young Kim, Seong Kyu Lee, Haing Woon Baik, Ki Ho Lee, Hyun Jin Kim, Kang Seo Park, Byung Joon Kim
- Korean Diabetes J. 2008;32(6):477-487. Published online December 1, 2008
- DOI: https://doi.org/10.4093/kdj.2008.32.6.477
- 2,053 View
- 23 Download
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The characteristic feature of pancreatic beta cells is highly developed endoplasmic reticulum (ER) due to a heavy engagement in insulin secretion. The ER serves several important function, including post-translational modification, folding, and assembly of newly synthesized secretory proteins, and its proper function is essential to cell survival. Various stress conditions can interfere with ER function. Pancreatic beta cells may be particularly vulnerable to ER stress that causes to impair insulin biosynthesis and beta cell survival through apoptosis. Glucagon like peptide-1 (GLP-1) is a new drug for treatment of type 2 diabetes and has effects on stimulation of insulin secretion and beta cell preservation. Also, it may have an antiapoptotic effect on beta cells, but detailed mechanisms are not proven. Therefore, we investigated the protective mechanism of GLP-1 in beta cells through ER stress response induced by 2-deoxy-D-glucose (2DG). METHODS: For induction of the ER stress, HIT-T15 cells (hamster beta cell line) were treated with 2DG (10 mM). Apoptosis was evaluated with MTT assay, hoechst 33342 staining and Annexin/PI flow cytometry. Expression of ER stress-related molecules was determined by real-time PCR or western blot. For blocking ER stress, we pretreated HIT-T15 cells with exendin-4 (Ex-4; GLP-1 receptor agonist) for 1 hour before stress induction. RESULTS: After induction with ER stress (2DG), beta cells were lost by apoptosis. We found that Ex-4 had a protective effect through ER stress related molecules (GRP78, GRP94, XBP-1, eIF2alpha, CHOP) modulation. Also, Ex-4 recovered the expression of insulin2 mRNA in beta cells. CONCLUSION: These results suggest that GLP-1 may protect beta cells apoptosis through ER stress modulation. -
Citations
Citations to this article as recorded by- Exendin-4 Protects Against Sulfonylurea-Induced β-Cell Apoptosis
Ju-Young Kim, Dong-Mee Lim, Hyung-Seo Park, Chan-Il Moon, Kyung-Jin Choi, Seong-Kyu Lee, Haing-Woon Baik, Keun-Young Park, Byung-Joon Kim
Journal of Pharmacological Sciences.2012; 118(1): 65. CrossRef - GLP-1 Can Protect Proinflammatory Cytokines Induced Beta Cell Apoptosis through the Ubiquitination
Dong Mee Lim, Ju Young Kim, Kang Woo Lee, Keun Young Park, Byung Joon Kim
Endocrinology and Metabolism.2011; 26(2): 142. CrossRef - Exendin-4 Protects Oxidative Stress-Induced β-Cell Apoptosis through Reduced JNK and GSK3β Activity
Ju-Young Kim, Dong-Mee Lim, Chan Il Moon, Kyung-Jin Jo, Seong-Kyu Lee, Haing-Woon Baik, Ki-Ho Lee, Kang-Woo Lee, Keun-Young Park, Byung-Joon Kim
Journal of Korean Medical Science.2010; 25(11): 1626. CrossRef
- Exendin-4 Protects Against Sulfonylurea-Induced β-Cell Apoptosis
- The Protective Effect of EGCG on INS-1 Cell in the Oxidative Stress and Mechanism.
- Mi Kyung Kim, Hye Sook Jung, Chang Shin Yoon, Min Jeong Kwon, Kyung Soo Koh, Byung Doo Rhee, Jeong Hyun Park
- Korean Diabetes J. 2008;32(2):121-130. Published online April 1, 2008
- DOI: https://doi.org/10.4093/kdj.2008.32.2.121
- 2,051 View
- 24 Download
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Oxidative stress is important in both diabetic complications and the development and the progression of type 2 diabetes via the effects on the pancreatic beta-cells. EGCG (epigallocatechin galleate), a major constituent of green tea, has been known to have beneficial effects on various diseases through the mechanisms of antioxidant and cell signaling modulation. But, very small numbers of studies were published about the direct effects of EGCG on the pancreatic beta cell lines. We performed this study to see the protective effect of EGCG on pancreatic beta cell line under H2O2 and the mechanisms of this phenomenon. METHODS: We used INS-1 cells and hydrogen peroxide as an oxidative stressor. Their viabilities were verified by MTT assay and FACS. The activity of glutathione peroxidase was assessed by total glutathione quantification kit. Western blot and semi-quantitative RT-PCR for the catalase, SOD (superoxide dismutase), PI3K and Akt were performed. Functional status of INS-1 cells was tested by GSIS (glucose stimulated insulin secretion). RESULTS: The biological effects of EGCG were different according to its concentrations. 10 micrometer EGCG effectively protected hydrogen peroxide induced damage in INS-1 cells. The expression and the activity of SOD, catalase and the glutathione peroxidase were significantly increased by EGCG. EGCG significantly increased PI3K and Akt activity and its effect was inhibited partially by wortmannin. GSIS was well preserved by EGCG. CONCLUSION: EGCG in low concentration effectively protected INS-1 cells from the oxidative stress through the activation of both antioxidant systems and anti-apoptosis signaling. Further studies will be necessary for the more detailed mechanisms and the clinical implications. -
Citations
Citations to this article as recorded by- Suppressive Effects of Epigallocatechin Gallate Pretreatment on the Expression of Inflammatory Cytokines in RAW264.7 Cells Activated by Lipopolysaccharide
Eun Ji Seo, Jun Go, Ji Eun Kim, Eun Kyoung Koh, Sung Hwa Song, Ji Eun Sung, Chan Kyu Park, Hyun Ah Lee, Dong Seob Kim, Hong Joo Son, Cung Yeoul Lee, Hee Seob Lee, Dae Youn Hwang
Journal of Life Science.2015; 25(9): 961. CrossRef - The Protective Effects of Chrysanthemum cornarium L. var. spatiosum Extract on HIT-T15 Pancreatic β-Cells against Alloxan-induced Oxidative Stress
In-Hye Kim, Kang-Jin Cho, Jeong-Sook Ko, Jae-Hyun Kim, Ae-Son Om
The Korean Journal of Food And Nutrition.2012; 25(1): 123. CrossRef - Protective Effects of Sasa Borealis Leaves Extract on High Glucose-Induced Oxidative Stress in Human Umbilical Vein Endothelial Cells
Ji-Young Hwang, Ji-Sook Han
Journal of the Korean Society of Food Science and Nutrition.2010; 39(12): 1753. CrossRef
- Suppressive Effects of Epigallocatechin Gallate Pretreatment on the Expression of Inflammatory Cytokines in RAW264.7 Cells Activated by Lipopolysaccharide
- The Effect of Alpha-lipoic Acid on the Cell Cycle Arrest and Apoptosis in Rat Vascular Smooth Muscle Cells.
- Hye Jin Kim, In Kyu Lee, Young Ho Kim, Soon Young Shin, Young Han Lee, Jung Guk Kim, Bo Wan Kim, Hye Soon Kim, Mi Kyoung Kim, Keun Gyu Park, Seong Yeol Ryu
- Korean Diabetes J. 2007;31(3):200-207. Published online May 1, 2007
- DOI: https://doi.org/10.4093/jkda.2007.31.3.200
- 2,138 View
- 20 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The proliferation of vascular smooth muscle cells (VSMCs) is a hallmark of atheroscelrosis and post-angioplasty restenosis. We previously showed that alpha-lipoic acid (ALA) inhibited neointimal hyperplasia and has potential anti-atherosclerosis effect in rat carotid artery balloon injured model. Here, we investigated whether alpha-lipoic acid inhibited proliferation of cells and induced apoptosis in rat vascular smooth muscle cells. METHODS: VSMCs were treated with ALA under each condition, harvested and protein was extracted. Same amount of protein was loaded into SDS-PAGE and western blot analysis was performed with various cell cycle regulation protein. To examine ALA induce apoptosis in VSMCs, FACS and DNA fragmentation assay were performed. Antioxidant effect of ALA was determined by DCF-DA staining. RESULTS: ALA induced VSMCs cell cycle arrest and induced p21, p27 and p53 proteins. Also ALA induced PTEN expression and AMPK phosphorylation. Increased AMPK phosphorylation reduced Erk-2 phosphorylation and finally arrested cell cycle promotion. The apoptotic effect was also shown by ALA treatment. Also we confirmed that ALA reduced ROS generation in VSMCs. CONCLUSION: The present data suggest that ALA has anti-proliferative effect and arrests cell proliferation. Therefore, ALA may provide new strategies for the prevention of neointimal hyperplasia after angioplasty.
- Mechanism of 2-Deoxy-D-ribose-induced Damage in Pancreatic beta-cells.
- Gwanpyo Koh, Jeong taek Woo, Dae Ho Lee, Seungjoon Oh, Sung Woon Kim, Jin Woo Kim, Young Seol Kim, Deok Bae Park
- Korean Diabetes J. 2007;31(2):105-112. Published online March 1, 2007
- DOI: https://doi.org/10.4093/jkda.2007.31.2.105
- 2,060 View
- 21 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Mechanism for glucose toxicity is known to be an increased oxidative stress produced by multiple pathways. In our previous report, 2-deoxy-d-ribose (dRib) promoted apoptosis by increasing oxidative stress in a pancreatic beta-cell line. We performed this study to investigate the mechanism of dRib-induced damage of beta-cells. METHODS: HIT-T15 cells were cultured in RPMI-1640 medium with 40 mM dRib for 24 hours after pretreatment with various concentrations of a metal chelator (DTPA) and inhibitors of protein glycation (aminoguanidine and pyridoxamine). Cell viability was determined by MTT assay. Apoptosis was analyzed by flow cytometry with annexin V/PI double staining. RESULTS: DTPA, which inhibits the monosaccharide autoxidation, partially reversed dRib-induced cytotoxicity in a dose-dependent manner (P < 0.01). The cytotoxicity was also suppressed dose-dependently by aminoguanidine (AG) and pyridoxamine (PM) (P < 0.05 and P < 0.01, repectively). Flow cytometric analysis showed that pretreatment of DTPA and AG also reversed the dRib-triggered apoptosis in a dose-dependent manner. We assessed the additional protective effects of inhibitors of protein glycation from dRib-induced cytotoxiciy in the presence of a metal chelator. The additions of AG (P < 0.05) and PM (P < 0.01) significantly reduced the cytotoxicity compared with DTPA alone group. CONCLUSION: This results suggest that dRib produce cytotoxicity and apoptosis through the mechanisms of advanced glycation endproducts (AGEs) formation including the monsaccharide autoxidation and protein glycation in pancreatic beta-cell. Thus, dRib could be a surrogate for glucose in the study of glucose toxicity and chronic diabetic complications. -
Citations
Citations to this article as recorded by- Isolation of Citrus Peel Flavonoid Bioconversion Microorganism and Inhibitory Effect on the Oxidative Damage in Pancreatic Beta Cells
Chi-Deok Park, Hee-Kyung Jung, Chang-Ho Park, Yoo-Seok Jung, Joo-Heon Hong, Hee-Sun Ko, Dong-Hee Kang, Hyun-Soo Kim
KSBB Journal.2012; 27(1): 67. CrossRef - Kaempferol protects HIT‐T15 pancreatic beta cells from 2‐deoxy‐D‐ribose‐induced oxidative damage
Yun Jung Lee, Kwang Sik Suh, Moon Chan Choi, Suk Chon, Seungjoon Oh, Jeong‐Taek Woo, Sung‐Woon Kim, Jin‐Woo Kim, Young Seol Kim
Phytotherapy Research.2010; 24(3): 419. CrossRef
- Isolation of Citrus Peel Flavonoid Bioconversion Microorganism and Inhibitory Effect on the Oxidative Damage in Pancreatic Beta Cells
- The Effect of High Glucose and TGF-beta on the Cellular Injury in Cultured Glomerular Epithelial Cells.
- Gui Hwa Jeong, Sung Chang Chung, Eui Dal Jung, Yun Jeong Doh, Hee Kyoung Kim, Soon Hong Park, In Hae Park, Jung Guk Kim, Sung Woo Ha, Bo Wan Kim, In Kyu Lee, Cheol Woo Ko
- Korean Diabetes J. 2006;30(4):254-263. Published online July 1, 2006
- DOI: https://doi.org/10.4093/jkda.2006.30.4.254
- 1,814 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The glomerulus is a complex physiological structure, as well as selective filtration barrier in the control of renal blood flow and blood pressure. Glomerular epithelial cells may play an important role in development of diabetic nephropathy. Apoptosis of the glomerular epithelial cells are characterized by disappearance of a selective filtration barrier. TGF-beta is a key factor in the development of diabetic nephropathy because of its effects on the accumulation of extracellular matrix and mesangial cell proliferation. We examined whether the high glucose and TGF-beta induce the apoptosis in cultured rat glomerular epithelial cells. METHODS: Glomerular epithelial cells were cultured from rat glomeruli and conditioned with different concentration of TGF-beta or high-glucose. We measured apoptosis of cultured rat glomerular epithelial cell conditioning with different concentration of TGF-beta or high-glucose by using DNA electrophoresis. RESULTS: High glucose (25 mM) induced apoptosis of cultured rat glomerular epithelial cells compared to controls. TGF-beta also induced cell death of cultured rat glomerular epithelial cells in dose dependent manner. CONCLUSION: These results suggest that high glucose and TGF-beta-induced cell death of glomerular epithelial cell may play an important role in diabetic nephropathy and proteinuria. Pathway of apoptosis or cell death by high glucose and TGF-beta must be investigated in the glomerular epithelial cells.
- Protective Effect of PGC-1 on Lipid Overload-induced Apoptosis in Vascular Endothelial Cell.
- Eun Hee Koh, Youn Mi Kim, Ha Jung Kim, Woo Je Lee, Jong Chul Won, Min Seon Kim, Ki Up Lee, Joong Yeol Park
- Korean Diabetes J. 2006;30(3):151-160. Published online May 1, 2006
- DOI: https://doi.org/10.4093/jkda.2006.30.3.151
- 2,083 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Fatty acids contribute to endothelial cell dysfunction and apoptosis by inducing accumulation of long chain fatty acyl CoA (LCAC), which increases oxidative stress in vascular endothelial cells. Forced expression of PGC-1 was shown to induce mitochondrial biogenesis and to control expression of mitochondrial enzymes involved in fatty acid oxidation. This study was undertaken to test the hypothesis that PGC-1 overexpression could prevent endothelial cell apoptosis by enhancing fatty acid oxidation and relieving oxidative stress in vascular endothelium. METHODS: Adenoviruses containing human PGC-1 (Ad-PGC-1) and beta-galactosidase (Ad-beta-gal) were transfected to confluent human aortic endothelial cells (HAECs). To investigate the effect of adenoviral PGC-1 gene transfer on apoptosis, combined treatment of linoleic acid (LA), an unsaturated fatty acid, was performed. RESULTS: PGC-1 overexpression inhibited the increase in ROS production and apoptosis of HAECs induced by LA. Also, PGC-1 led to a significant increase in fatty acid oxidation and decrease in triglyceride content in HAECs. LA caused the decrease of adenine nucleotide translocase (ANT) activity and transient mitochondrial hyperpolarization, which was followed by depolarization. PGC-1 overexpression prevented these processes. CONCLUSION: In summary, PGC-1 overexpression inhibited mitochondrial dysfunction and apoptosis by facilitating fatty acid oxidation and protecting against the damage from oxidative stress in HAECs. The data collectively suggest that the regulation of intracellular PGC-1 expression might play a critical role in preventing atherosclerosis.
- The Effect of cAMP-Elevating Agents on High Glucose-Induced Apoptosis of Isolated Islets of Rat Pancreas.
- Gwan Pyo Koh, Kwang Sik Suh, Suk Chon, Seung Joon Oh, Jeong Taek Woo, Sung Woon Kim, Jin Woo Kim, Young Seol Kim, Sun Hee Kwon
- Korean Diabetes J. 2004;28(6):490-500. Published online December 1, 2004
- 1,059 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
High glucose-induced apoptosis has been implicated in the loss of beta-cells of the pancreatic islets in animal models of type 2 diabetes. GLP-1 has been shown to reduce apoptosis by the cAMP-dependent mechanism in beta-cells. Other studies have also shown that elevated levels of intracellular cyclic AMP delayed apoptosis in other types of cells. We investigated whether cAMP-elevating agents could suppress the high glucose-induced apoptosis of isolated rat islets. METHODS: Pancreatic islets were isolated from Sprague-Dawley (SD) rats. The expression of phosphodiesterase (PDE) 3 subtypes was investigated by using extracts of freshly isolated islets and analyzing them by RT-PCR. After 2 days of isolation, the islets were cultured in RPMI-1640 media containing 5% FBS with various glucose concentrations (11.1, 16.7 and 27.8 mM), 5x10-6 M forskolin, 2x10-4 M 3-isobutyl-1-methylxanthine (IBMX), 10-5 M cilostazol, and 10-6, 5x10-6 and 10-5 M H-89 for 5 days. The islet apoptosis was measured by a sandwich enzyme-immunoassay using antihistone antibody. RESULTS: Apoptosis was lowest at 11.1 mM glucose concentration, and increased at higher glucose concentrations (1.00 +/- 0.04 A.U. (arbitrary unit) at 11.1 mM, 1.17 +/- 0.12 A.U. at 16.7 mM, and 1.65 +/-0.13 A.U. at 27.8 mM (P <0.05 for 11.1 mM). Both PDE 3A and 3B mRNA were expressed in the islet extracts. In 16.7 and 27.8 mM glucose concentrations, forskolin (P <0.01), IBMX (P <0.05) and cilostazol (P < 0.05) suppressed apoptosis of the islet cells. Protein kinase A (PKA) nhibitor, H-89, did not prevent the inhibition of apoptosis by forskolin. CONCLUSION: These results show that high glucose-induced apoptosis of the cells in rat islet is attenuated by such cAMP-elevating agents as cilostazol. However, cyclic AMP regulation of islet apoptosis may occur via a PKA-independent signaling pathway.
- Study on the Methylglyoxal-induced Apoptosis in Bovine Retinal Pericytes.
- Jaetaek Kim, Seok Hong Lee, Jang Won Son, Jeong An Lee, Yeon Sahng Oh, Soon Hyun Shinn
- Korean Diabetes J. 2004;28(3):199-207. Published online June 1, 2004
- 817 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
One of the histopathological hallmarks of early diabetic retinopathy is the loss of pericytes. Evidences suggest that this pericyte loss in vivo is mediated by apoptosis. However, the underlying cause of pericyte apoptosis is not fully understood. This study investigated the influence of methylglyoxal(MGO), a reactive alpha-dicarbonyl compound of glucose metabolism, on the apoptotic cell death in retinal pericytes. METHODS: Primary cultures of retinal pericytes were prepared from isolated bovine retinal microvessels. The cells were incubated under normoglycemic conditions after treatment with 200-800muM methylglyoxal for 6 hours. The cell viability was assessed using the MTT assay. The apoptosis and intracellular reactive oxygen species(ROS) generation were measured using an ELISA kit and flow cytometry, respectively. The NF-kappaB activation was detected by immunocytochemistry. RESULTS: MGO produced a progressive cytotoxic effect on the retinal pericytes. An analysis of the internucleosomal DNA fragmentation by ELISA showed that MGO(200 to 800muM) induced apoptosis in a concentration-dependent manner. ROS were generated earlier and the antioxidant, N-acetyl cysteine, inhibited the MGO-induced apoptosis. The NF-kappaB activation and increased caspase-3 activity were detected. The apoptosis was also inhibited by the caspase-3 inhibitor, Z-DEVD-fmk, or the NF-kappaB inhibitor, pyrrolidine dithiocarbamate. CONCLUSION: These results suggest that the elevated MGO levels observed in diabetes may cause apoptosis in the retinal pericytes through an oxidative stress mechanism, and suggests that the nuclear activation of NF-kappaB is involved in the apoptotic process.

 KDA
KDA
 First
First Prev
Prev





