
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Previous issues
- Page Path
- HOME > Browse > Previous issues
- Pathophysiology
- Hyperinsulinemia in Obesity, Inflammation, and Cancer
- Anni M.Y. Zhang, Elizabeth A. Wellberg, Janel L. Kopp, James D. Johnson
- Diabetes Metab J. 2021;45(3):285-311. Published online March 29, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0250
- Correction in: Diabetes Metab J 2021;45(4):622
- 23,121 View
- 901 Download
- 81 Web of Science
- 88 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub 
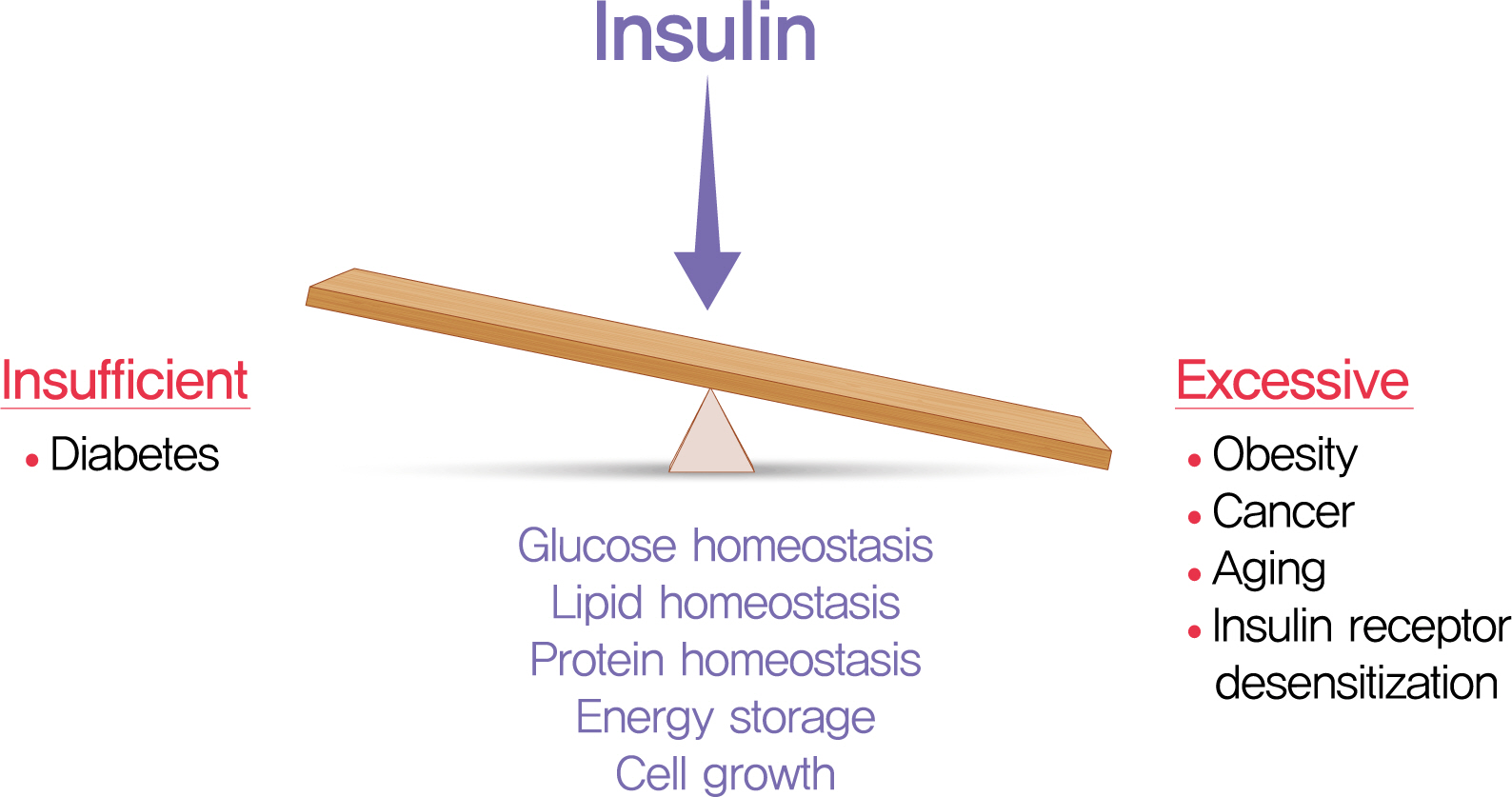
- The relative insufficiency of insulin secretion and/or insulin action causes diabetes. However, obesity and type 2 diabetes mellitus can be associated with an absolute increase in circulating insulin, a state known as hyperinsulinemia. Studies are beginning to elucidate the cause-effect relationships between hyperinsulinemia and numerous consequences of metabolic dysfunctions. Here, we review recent evidence demonstrating that hyperinsulinemia may play a role in inflammation, aging and development of cancers. In this review, we will focus on the consequences and mechanisms of excess insulin production and action, placing recent findings that have challenged dogma in the context of the existing body of literature. Where relevant, we elaborate on the role of specific signal transduction components in the actions of insulin and consequences of chronic hyperinsulinemia. By discussing the involvement of hyperinsulinemia in various metabolic and other chronic diseases, we may identify more effective therapeutics or lifestyle interventions for preventing or treating obesity, diabetes and cancer. We also seek to identify pertinent questions that are ripe for future investigation.
-
Citations
Citations to this article as recorded by- High adherence to Western dietary pattern increases breast cancer risk (an EPIC-Spain study)
Adela Castelló, Miguel Rodríguez-Barranco, Virginia Lope, Marcela Guevara, Sandra Colorado-Yohar, Ane Dorronsoro, José Ramón Quirós, Carlota Castro-Espin, Carmen Sayon-Orea, Carmen Santiuste, Pilar Amiano, Cristina Lasheras, María-José Sanchez, Marina Pol
Maturitas.2024; 179: 107868. CrossRef - Dietary Diabetes Risk Reduction Score (DDRRS) and Breast Cancer Risk: A Case-Control Study in Iran
Milad Mohammadzadeh, Alireza Bahrami, Fatemeh Abdi, Fatemeh Ghafouri-Taleghani, Amin Paydareh, Saba Jalali, Zeinab Heidari, Bahram Rashidkhani
Nutrition and Cancer.2024; 76(1): 106. CrossRef - White adipocyte dysfunction and obesity-associated pathologies in humans
Carolina E. Hagberg, Kirsty L. Spalding
Nature Reviews Molecular Cell Biology.2024; 25(4): 270. CrossRef - Cumulative exposure to impaired fasting glucose and gastrointestinal cancer risk: A nationwide cohort study
Byeong Yun Ahn, Bokyung Kim, Sanghyun Park, Sang Gyun Kim, Kyungdo Han, Soo‐Jeong Cho
Cancer.2024;[Epub] CrossRef - Leptin receptor deficiency impedes metabolic surgery related-weight loss through inhibition of energy expenditure in db/db mice
Dan Tong, Jie Xiang, Wei Liu, Fang Sun, Lijuan Wang, Aidi Mou, Tingbing Cao, Qing Zhou, Mei You, Yingying Liao, Peng Gao, Daoyan Liu, Zongshi Lu, Zhiming Zhu
Diabetology & Metabolic Syndrome.2024;[Epub] CrossRef - Obesity and Cancer Rehabilitation for Functional Recovery and Quality of Life in Breast Cancer Survivors: A Comprehensive Review
Lorenzo Lippi, Alessandro de Sire, Arianna Folli, Alessio Turco, Stefano Moalli, Marco Marcasciano, Antonio Ammendolia, Marco Invernizzi
Cancers.2024; 16(3): 521. CrossRef - Exploring the Role of Hyperinsulinemia in Obesity-Associated Tumor Development
Ericka Vélez-Bonet, Kristyn Gumpper-Fedus, Zobeida Cruz-Monserrate
Cancer Research.2024; 84(3): 351. CrossRef - Exploring Promising Therapies for Non-Alcoholic Fatty Liver Disease: A ClinicalTrials.gov Analysis
Omar Hegazi, Samer Alalalmeh, Moyad Shahwan, Ammar Jairoun, Mansour Alourfi, Ghfran Bokhari, Abdullah Alkhattabi, Saeed Alsharif, Mohannad Aljehani, Abdulmalik Alsabban, Mohammad Almtrafi, Ysear Zakri, Abdullah AlMahmoud, Khalid Alghamdi, Ahmed Ashour, Na
Diabetes, Metabolic Syndrome and Obesity.2024; Volume 17: 545. CrossRef - Predictors of Cardiac Autonomic Dysfunction in Obesity-Related Hypertension
Aqsa Mujaddadi, Saima Zaki, Majumi M Noohu, Irshad Husain Naqvi, Zubia Veqar
High Blood Pressure & Cardiovascular Prevention.2024; 31(1): 77. CrossRef - The obesity-autophagy-cancer axis: Mechanistic insights and therapeutic perspectives
Amir Barzegar Behrooz, Marco Cordani, Alessandra Fiore, Massimo Donadelli, Joseph W. Gordon, Daniel J. Klionsky, Saeid Ghavami
Seminars in Cancer Biology.2024; 99: 24. CrossRef - Metformin’s role in lowering colorectal cancer risk among individuals with diabetes from the Southern Community Cohort Study
Thomas Lawler, Zoe L. Walts, Lauren Giurini, Mark Steinwandel, Loren Lipworth, Harvey J. Murff, Wei Zheng, Shaneda Warren Andersen
Cancer Epidemiology.2024; 90: 102566. CrossRef - Sex differences in insulin regulation of skeletal muscle glycogen synthase and changes during weight loss and exercise in adults
Alice S. Ryan, Guoyan Li, Shawna McMillin, Heidi K. Ortmeyer
Obesity.2024; 32(4): 667. CrossRef - Therapeutic Repurposing of Antidiabetic Drugs in Diabetes-associated
Comorbidities
Kalyani Pathak, Manash Pratim Pathak, Riya Saikia, Urvashee Gogoi, Ratna Jyoti Das, Pompy Patowary, Partha Pratim Kaishap, Smita Bordoloi, Jyotirmoy Das, Himangshu Sarma, Mohammad Zaki Ahmad, Aparoop Das
Current Drug Therapy.2024; 19(2): 178. CrossRef - The effect of metabolic syndrome on prognosis of diffuse large B-cell lymphoma
Wenjing Xiong, Liru Li, Xue Hui, Yue Liu, Hongbin Li, Yue Zhang, Shu Zhao
Clinical and Translational Oncology.2024;[Epub] CrossRef - Impact of Obesity-Related Endoplasmic Reticulum Stress on Cancer and Associated Molecular Targets
Joud AlBashtawi, Hend Al-Jaber, Sara Ahmed, Layla Al-Mansoori
Biomedicines.2024; 12(4): 793. CrossRef - Healthcare Management of an Obese Person
Syeda Rida Baqir, Shafaque Aslam Khan, Bushra Marium Zaman, Tahira Hamid Ali, Nazish Saeed Bangash, Muhammad Amjad Ali, Fatima Zaidi, Jahan Ara Farooq
DIET FACTOR (Journal of Nutritional and Food Sciences).2024; : 10. CrossRef - Associations between diabetes and cancer: A 10-year national population-based retrospective cohort study
Heléna Safadi, Ágnes Balogh, Judit Lám, Attila Nagy, Éva Belicza
Diabetes Research and Clinical Practice.2024; 211: 111665. CrossRef - Advances in “adiponcosis”: Insights in the inner mechanisms at the base of adipose and tumour tissues interplay
Cristina Pagano, Erika di Zazzo, Giorgio Avilia, Beatrice Savarese, Giovanna Navarra, Maria Chiara Proto, Donatella Fiore, Monica Rienzo, Patrizia Gazzerro, Chiara Laezza, Maurizio Bifulco
International Journal of Cancer.2023; 152(12): 2464. CrossRef - Influence of antidiabetic drugs on glucose metabolism and immune response in patients with metastatic pancreatic ductal adenocarcinoma receiving gemcitabine plus nab-paclitaxel as first-line treatment
Andrea Pretta, Pina Ziranu, Riccardo Giampieri, Clelia Donisi, Erika Cimbro, Dario Spanu, Eleonora Lai, Federica Pecci, Francesca Balconi, Alessio Lupi, Marta Pozzari, Mara Persano, Sara Murgia, Valeria Pusceddu, Marco Puzzoni, Rossana Berardi, Mario Scar
Digestive and Liver Disease.2023; 55(5): 655. CrossRef - Islet amyloid polypeptide does not suppress pancreatic cancer
Austin J. Taylor, Evgeniy Panzhinskiy, Paul C. Orban, Francis C. Lynn, David F. Schaeffer, James D. Johnson, Janel L. Kopp, C. Bruce Verchere
Molecular Metabolism.2023; 68: 101667. CrossRef - Pathophysiology of obesity and its associated diseases
Xin Jin, Tingting Qiu, Li Li, Rilei Yu, Xiguang Chen, Changgui Li, Christopher G. Proud, Tao Jiang
Acta Pharmaceutica Sinica B.2023; 13(6): 2403. CrossRef - Metabolic syndrome as a risk factor for oncogenesis
M.A. Osadchuk, I.N. Vasilieva, V.V. Kozlov, O.I. Mitrokhina
Profilakticheskaya meditsina.2023; 26(1): 70. CrossRef - Polycystic ovary syndrome: Causes, symptoms, pathophysiology, and remedies
Ananya Chaudhuri
Obesity Medicine.2023; 39: 100480. CrossRef - Metformin and long non-coding RNAs in breast cancer
Morteza Gholami, Zeynab Nickhah Klashami, Pirooz Ebrahimi, Amir Ali Mahboobipour, Amir Salehi Farid, Aida Vahidi, Marziyeh Zoughi, Mojgan Asadi, Mahsa M. Amoli
Journal of Translational Medicine.2023;[Epub] CrossRef - Host-Related Factors in the Interplay among Inflammation, Immunity and Dormancy in Breast Cancer Recurrence and Prognosis: An Overview for Clinicians
Lorenzo Ruggieri, Anna Moretti, Rossana Berardi, Maria Silvia Cona, Davide Dalu, Cecilia Villa, Davide Chizzoniti, Sheila Piva, Anna Gambaro, Nicla La Verde
International Journal of Molecular Sciences.2023; 24(5): 4974. CrossRef - Degree of obesity and gastrointestinal adverse reactions influence the weight loss effect of liraglutide in overweight or obese patients with type 2 diabetes
Fang Zhou, Lu Jiang, Jiamei Guo, Yuting Fan, Qin Pan, Tianlian Li, Xiaoshi Sun, Ping Li
Therapeutic Advances in Chronic Disease.2023; 14: 204062232311615. CrossRef - Both early and late maternal age at childbirth is associated with increasing odds of central obesity in offspring
Hongyun Chen, Yinhua Feng, Changying Chen, Songcheng Yu
American Journal of Human Biology.2023;[Epub] CrossRef - Integrated Physiology of the Exocrine and Endocrine Compartments in Pancreatic Diseases: Workshop Proceedings
Teresa L. Mastracci, Minoti Apte, Laufey T. Amundadottir, Alexandra Alvarsson, Steven Artandi, Melena D. Bellin, Ernesto Bernal-Mizrachi, Alejandro Caicedo, Martha Campbell-Thompson, Zobeida Cruz-Monserrate, Abdelfattah El Ouaamari, Kyle J. Gaulton, Andre
Diabetes.2023; 72(4): 433. CrossRef - Obesity-induced changes in cancer cells and their microenvironment: Mechanisms and therapeutic perspectives to manage dysregulated lipid metabolism
Miriam Lee-Rueckert, Marina Canyelles, Mireia Tondo, Noemi Rotllan, Petri T. Kovanen, Vicenta Llorente-Cortes, Joan Carles Escolà-Gil
Seminars in Cancer Biology.2023; 93: 36. CrossRef - PIK3CA mutation in endometriotic epithelial cells promotes viperin-dependent inflammatory response to insulin
Mike R. Wilson, Shannon Harkins, Jake J. Reske, Rebecca A. Siwicki, Marie Adams, Victoria L. Bae-Jump, Jose M. Teixeira, Ronald L. Chandler
Reproductive Biology and Endocrinology.2023;[Epub] CrossRef - Gut microbiome modified by bariatric surgery improves insulin sensitivity and correlates with increased brown fat activity and energy expenditure
Jitender Yadav, Tao Liang, Tairan Qin, Nayanan Nathan, Katherine J.P. Schwenger, Lauren Pickel, Li Xie, Helena Lei, Daniel A. Winer, Heather Maughan, Susan J. Robertson, Minna Woo, Wendy Lou, Kate Banks, Timothy Jackson, Allan Okrainec, Susy S. Hota, Susa
Cell Reports Medicine.2023; 4(5): 101051. CrossRef - Prevalence of Type 2 Diabetes, Impaired Fasting Glucose, and Diabetes Risk in an Adult and Older North-Eastern Portuguese Population
Pedro M. Magalhães, José E. Teixeira, João P. Bragada, Carlos M. Duarte, José A. Bragada
Healthcare.2023; 11(12): 1712. CrossRef - Hyperinsulinemia Influences the Short-Term Efficiency of Laparoscopic Sleeve Gastrectomy for Patients with Obesity and Insulin Resistance
Zilong Yue, Long Qian, Yan Jin, Yabin Xia, Hui Sha, Qin Wu, Kaifeng Hu
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 1745. CrossRef - Acetyl-11-Keto-Beta-Boswellic Acid Has Therapeutic Benefits for NAFLD Rat Models That Were Given a High Fructose Diet by Ameliorating Hepatic Inflammation and Lipid Metabolism
Reza Ataei Kachouei, Alireza Doagoo, Maral Jalilzadeh, Seyyed Hossein Khatami, Shima Rajaei, Ali Jahanbazi Jahan-Abad, Farzaneh Salmani, Roya Pakrad, Somayeh Mahmoodi Baram, Mitra Nourbakhsh, Mohammad-Amin Abdollahifar, Hojjat Allah Abbaszadeh, Shokoofeh
Inflammation.2023; 46(5): 1966. CrossRef - Cancer Risk and its Association With Diabetes Mellitus in Patients With Acromegaly: A Two Center-based Study
Zhe-Hao Xiao, Cheng Wang, Yong Wang, Shang-Kun Yuan, Cheng Huang, Ren-Fang Chen, Yong Li
Endocrine Practice.2023; 29(9): 699. CrossRef - Obesity, diabetes, and cancer: epidemiology, pathophysiology, and potential interventions
Leonardo de Andrade Mesquita, Laura Fink Wayerbacher, Gilberto Schwartsmann, Fernando Gerchman
Archives of Endocrinology and Metabolism.2023;[Epub] CrossRef - Insulin Stimulates IL-23 Expression in Human Adipocytes: A Possible Explanation for the Higher Prevalence of Psoriasis in Obesity
Angelo Di Vincenzo, Marnie Granzotto, Marika Crescenzi, Camilla Costa, Stefano Piaserico, Vincenzo Vindigni, Roberto Vettor, Marco Rossato
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 1885. CrossRef - Immunometabolic aspects of chronic nonspecific inflammation in obesity
O. V. Skvortsova, N. B. Migacheva, E. G. Mikhailova
Meditsinskiy sovet = Medical Council.2023; (12): 75. CrossRef - The Reponic Classification of Insulin
Joshua Moen
Canadian Journal of Diabetes.2023; 47(8): 680. CrossRef - Dietary Acid Load and Cancer Risk: A Review of the Uruguayan Experience
Alvaro Luis Ronco, Maximilian Andreas Storz
Nutrients.2023; 15(14): 3098. CrossRef - Non-alcoholic fatty liver disease and diabetes mellitus as growing aetiologies of hepatocellular carcinoma
Stephanie Talamantes, Michela Lisjak, Eduardo H. Gilglioni, Camilo J. Llamoza-Torres, Bruno Ramos-Molina, Esteban N. Gurzov
JHEP Reports.2023; 5(9): 100811. CrossRef - Alteration of Metabolic Syndrome Is Associated with the Decreased Risk of Colorectal Cancer
Eun Hyo Jin, Yoon Jin Choi, Joo Hyun Lim, Cheol Min Shin, Kyungdo Han, Dong Ho Lee
Journal of Clinical Medicine.2023; 12(15): 4889. CrossRef - Dietary interventions improve diabetic kidney disease, but not peripheral neuropathy, in a db/db mouse model of type 2 diabetes
Stephanie A. Eid, Phillipe D. O'Brien, Katharina H. Kretzler, Dae‐Gyu Jang, Faye E. Mendelson, John M. Hayes, Andrew Carter, Hongyu Zhang, Subramaniam Pennathur, Frank C. Brosius, Emily J. Koubek, Eva L. Feldman
The FASEB Journal.2023;[Epub] CrossRef - Decoding the role of leptin and adiponectin in obesity-related gastrointestinal cancer
Vanda Marques, Fabiola Arella, Marta B. Afonso, André A. Santos, Cecília M.P. Rodrigues
Clinical Science.2023; 137(15): 1095. CrossRef - Metformin Can Attenuate Beta-Cell Hypersecretion—Implications for Treatment of Children with Obesity
Quan Wen, Rasmus Stenlid, Azazul Islam Chowdhury, Iris Ciba, Banu Aydin, Sara Y. Cerenius, Hannes Manell, Anders Forslund, Peter Bergsten
Metabolites.2023; 13(8): 917. CrossRef - The Effectiveness of a Low Glycemic Index/Load Diet on Cardiometabolic, Glucometabolic, and Anthropometric Indices in Children with Overweight or Obesity: A Systematic Review and Meta-Analysis
Ioustini Kalaitzopoulou, Xenophon Theodoridis, Evangelia Kotzakioulafi, Kleo Evripidou, Michail Chourdakis
Children.2023; 10(9): 1481. CrossRef - A review of the last decade: pancreatic cancer and type 2 diabetes
Jiaqi Wu, Liang Tang, Feng Zheng, Xun Chen, Lei Li
Archives of Physiology and Biochemistry.2023; : 1. CrossRef - Characteristics of Children and Adolescents with Hyperinsulinemia Undergoing Oral Glucose Tolerance Test: A Single-Center Retrospective Observational Study
Clelia Cipolla, Ilaria Lazzareschi, Antonietta Curatola, Claudia Lasorella, Lucia Celeste Pane, Linda Sessa, Giulia Rotunno, Donato Rigante, Giorgio Sodero
Diseases.2023; 11(3): 110. CrossRef - Hyperinsulinemia acts via acinar insulin receptors to initiate pancreatic cancer by increasing digestive enzyme production and inflammation
Anni M.Y. Zhang, Yi Han Xia, Jeffrey S.H. Lin, Ken H. Chu, Wei Chuan K. Wang, Titine J.J. Ruiter, Jenny C.C. Yang, Nan Chen, Justin Chhuor, Shilpa Patil, Haoning Howard Cen, Elizabeth J. Rideout, Vincent R. Richard, David F. Schaeffer, Rene P. Zahedi, Chr
Cell Metabolism.2023; 35(12): 2119. CrossRef - Dairy Intake Modifies the Level of the Bile Acid Precursor and Its Correlation with Serum Proteins Associated with Cholesterol Clearance in Subjects with Hyperinsulinemia
Atena Mahdavi, Jocelyn Trottier, Olivier Barbier, Michel Lebel, Iwona Rudkowska
Nutrients.2023; 15(22): 4707. CrossRef - Trapa Bispinosa Roxb. Inhibits the Insulin-Dependent AKT/WNK1 Pathway to Induce Autophagy in Mice with Type 2 Diabetes
Takahiro Suzuki, Takehito Sato, Kaori Masuhara, Mizuki Tokusanai, Hisako Akatsuka, Tomohiro Kashikawa, Yasuyuki Suzuki
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 3095. CrossRef - Effects of Functional and Nutraceutical Foods in the Context of the Mediterranean Diet in Patients Diagnosed with Breast Cancer
Giovanna Flore, Andrea Deledda, Mauro Lombardo, Andrea Armani, Fernanda Velluzzi
Antioxidants.2023; 12(10): 1845. CrossRef - A systematic review exploring the mechanisms by which citrus bioflavonoid supplementation benefits blood glucose levels and metabolic complications in type 2 diabetes mellitus
Ankit Gupta, Abdulsatar Jamal, Dina A. Jamil, Hayder A. Al-Aubaidy
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(11): 102884. CrossRef - Diabetes Mellitus and Gastric Cancer: Correlation and Potential Mechanisms
Li Wang, Zhe Zhang, Eusebio Chiefari
Journal of Diabetes Research.2023; 2023: 1. CrossRef - EXPLORATORY ANALYSIS OF DIETARY PATTERNS OF PATIENTS WITH GASTRIC ADENOCARCINOMA: A CASE-CONTROL STUDY IN CENTRAL BRAZIL
Silvana Barbosa SANTIAGO, Gabriela Rodrigues de SOUSA, Amanda Ferreira Paes Landim RAMOS, Gisele Aparecida FERNANDES, Maria Paula CURADO, Mônica Santiago BARBOSA
Arquivos de Gastroenterologia.2023; 60(4): 419. CrossRef - Risk of cancer in acromegaly patients: An updated meta-analysis and systematic review
Zhehao Xiao, Pingping Xiao, Yong Wang, Chen Fang, Yong Li, Alvaro Galli
PLOS ONE.2023; 18(11): e0285335. CrossRef - Long Follow-Up Times Weaken Observational Diet–Cancer Study Outcomes: Evidence from Studies of Meat and Cancer Risk
William B. Grant
Nutrients.2023; 16(1): 26. CrossRef - Serum Lipid Profiles and Cholesterol-Lowering Medication Use in Relation to Subsequent Risk of Colorectal Cancer in the UK Biobank Cohort
Fangcheng Yuan, Wanqing Wen, Guochong Jia, Jirong Long, Xiao-Ou Shu, Wei Zheng
Cancer Epidemiology, Biomarkers & Prevention.2023; 32(4): 524. CrossRef - Prevalence of diabetes mellitus among 80,193 gastrointestinal cancer patients in five European and three Asian countries
Christoph Roderburg, Sven H. Loosen, Laura Hoyer, Tom Luedde, Karel Kostev
Journal of Cancer Research and Clinical Oncology.2022; 148(5): 1057. CrossRef - The Intricate Crosstalk Between Insulin and Pancreatic Ductal Adenocarcinoma: A Review From Clinical to Molecular
Junyuan Deng, Yujie Guo, Jiali Du, Jichun Gu, Lei Kong, Boan Tao, Ji Li, Deliang Fu
Frontiers in Cell and Developmental Biology.2022;[Epub] CrossRef - Effects of hyperinsulinemia on pancreatic cancer development and the immune microenvironment revealed through single-cell transcriptomics
Anni M. Y. Zhang, Ken H. Chu, Brian F. Daly, Titine Ruiter, Yan Dou, Jenny C. C. Yang, Twan J. J. de Winter, Justin Chhuor, Su Wang, Stephane Flibotte, Yiwei Bernie Zhao, Xiaoke Hu, Hong Li, Elizabeth J. Rideout, David F. Schaeffer, James D. Johnson, Jane
Cancer & Metabolism.2022;[Epub] CrossRef - Ghrelin and Cancer: Examining the Roles of the Ghrelin Axis in Tumor Growth and Progression
Anuhya S. Kotta, Abigail S. Kelling, Karen A. Corleto, Yuxiang Sun, Erin D. Giles
Biomolecules.2022; 12(4): 483. CrossRef - Influence of obesity on reproductive health before andduring pregnancy
A. Konwisser, O. Korytko
INTERNATIONAL JOURNAL OF ENDOCRINOLOGY (Ukraine).2022; 18(8): 446. CrossRef - Research Status of the Correlation between Type 2 Diabetes Mellitus and the Occurrence and Development of Breast Cancer
聪 李
Advances in Clinical Medicine.2022; 12(04): 3010. CrossRef - Effect of Carbohydrate-Restricted Diets and Intermittent Fasting on Obesity, Type 2 Diabetes Mellitus, and Hypertension Management: Consensus Statement of the Korean Society for the Study of Obesity, Korean Diabetes Association, and Korean Society of Hype
Jong Han Choi, Yoon Jeong Cho, Hyun-Jin Kim, Seung-Hyun Ko, Suk Chon, Jee-Hyun Kang, Kyoung-Kon Kim, Eun Mi Kim, Hyun Jung Kim, Kee-Ho Song, Ga Eun Nam, Kwang Il Kim
Diabetes & Metabolism Journal.2022; 46(3): 355. CrossRef - Obesity and Pancreatic Cancer: Recent Progress in Epidemiology, Mechanisms and Bariatric Surgery
Shuhei Shinoda, Naohiko Nakamura, Brett Roach, David A. Bernlohr, Sayeed Ikramuddin, Masato Yamamoto
Biomedicines.2022; 10(6): 1284. CrossRef - Effect of carbohydrate-restricted diets and intermittent fasting on obesity, type 2 diabetes mellitus, and hypertension management: consensus statement of the Korean Society for the Study of obesity, Korean Diabetes Association, and Korean Society of Hype
Jong Han Choi, Yoon Jeong Cho, Hyun-Jin Kim, Seung-Hyun Ko, Suk Chon, Jee-Hyun Kang, Kyoung-Kon Kim, Eun Mi Kim, Hyun Jung Kim, Kee-Ho Song, Ga Eun Nam, Kwang Il Kim
Clinical Hypertension.2022;[Epub] CrossRef - Vitamin D and Risk of Obesity-Related Cancers: Results from the SUN (‘Seguimiento Universidad de Navarra’) Project
Rodrigo Sánchez-Bayona, Maira Bes-Rastrollo, Cesar I. Fernández-Lázaro, Maite Bastyr, Ainhoa Madariaga, Juan J. Pons, Miguel A. Martínez-González, Estefanía Toledo
Nutrients.2022; 14(13): 2561. CrossRef - Metabolically Defined Body Size Phenotypes and Risk of Endometrial Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC)
Nathalie Kliemann, Romain Ould Ammar, Carine Biessy, Audrey Gicquiau, Verena Katzke, Rudolf Kaaks, Anne Tjønneland, Anja Olsen, Maria-Jose Sánchez, Marta Crous-Bou, Fabrizio Pasanisi, Sandar Tin Tin, Aurora Perez-Cornago, Dagfinn Aune, Sofia Christakoudi,
Cancer Epidemiology, Biomarkers & Prevention.2022; 31(7): 1359. CrossRef - Effect of Carbohydrate-Restricted Diets and Intermittent Fasting on Obesity, Type 2 Diabetes Mellitus, and Hypertension Management: Consensus Statement of the Korean Society for the Study of Obesity, Korean Diabetes Association, and Korean Society of Hype
Jong Han Choi, Yoon Jeong Cho, Hyun-Jin Kim, Seung-Hyun Ko, Suk Chon, Jee-Hyun Kang, Kyoung-Kon Kim, Eun Mi Kim, Hyun Jung Kim, Kee-Ho Song, Ga Eun Nam, Kwang Il Kim
Journal of Obesity & Metabolic Syndrome.2022; 31(2): 100. CrossRef - Detangling the interrelations between MAFLD, insulin resistance, and key hormones
Shreya C. Pal, Mohammed Eslam, Nahum Mendez-Sanchez
Hormones.2022; 21(4): 573. CrossRef - Obesity, cancer risk, and time-restricted eating
Manasi Das, Nicholas J. G. Webster
Cancer and Metastasis Reviews.2022; 41(3): 697. CrossRef - Associations between early-life growth pattern and body size and follicular lymphoma risk and survival: a family-based case-control study
Michael K. Odutola, Marina T. van Leeuwen, Jennifer Turner, Fiona Bruinsma, John F. Seymour, H. Miles Prince, Samuel T. Milliken, Mark Hertzberg, Judith Trotman, Stephen S. Opat, Robert Lindeman, Fernando Roncolato, Emma Verner, Michael Harvey, Campbell T
Cancer Epidemiology.2022; 80: 102241. CrossRef - Differential expression of Ago2‐mediated microRNA signaling in adipose tissue is associated with food‐induced obesity
Hansi Zhang, Liang Qiao, Xiaoxuan Liu, Xiaojing Han, Jing Kang, Yanli Liu, Juntang Lin, Xin Yan
FEBS Open Bio.2022; 12(10): 1828. CrossRef - Proposal for standardizing normal insulin ranges in Brazilian patients and a new classification of metabolic syndrome
Pedro Renato Chocair, Precil Diego Miranda de Menezes Neves, Victor Augusto Hamamoto Sato, Sara Mohrbacher, Érico Souza Oliveira, Leonardo Victor Barbosa Pereira, Alessandra Martins Bales, Fagner Pereira da Silva, John A. Duley, Américo Lourenço Cuvello-N
Frontiers in Medicine.2022;[Epub] CrossRef - Two models of insulin resistance development and the strategy to combat age-related diseases: literature review
A. V. Martyushev-Poklad, D. S. Yankevich, M. V. Petrova, N. G. Savitskaya
Problems of Endocrinology.2022; 68(4): 59. CrossRef - In Vitro Mimicking of Obesity-Induced Biochemical Environment to Study Obesity Impacts on Cells and Tissues
Abdelaziz Ghanemi, Mayumi Yoshioka, Jonny St-Amand
Diseases.2022; 10(4): 76. CrossRef - Long‐term survival analysis of patients with stage IIIB‐IV non‐small cell lung cancer complicated by type 2 diabetes mellitus: A retrospective propensity score matching analysis
Xuejiao Li, Haiyan Fang, Dongwei Zhang, Liming Xia, Xiang Wang, Jingping Yang, Shaohu Zhang, Ya Su, Yongfu Zhu
Thoracic Cancer.2022; 13(23): 3268. CrossRef - Diabetes and skin cancers: Risk factors, molecular mechanisms and impact on prognosis
Elena-Codruta Dobrică, Madalina Laura Banciu, Vincent Kipkorir, Mohammad Amin Khazeei Tabari, Madeleine Jemima Cox, L V Simhachalam Kutikuppala, Mihnea-Alexandru Găman
World Journal of Clinical Cases.2022; 10(31): 11214. CrossRef - The role of the mTOR pathway in diabetic retinopathy
Fabio Casciano, Enrico Zauli, Erika Rimondi, Marco Mura, Maurizio Previati, Massimo Busin, Giorgio Zauli
Frontiers in Medicine.2022;[Epub] CrossRef - Hyperinsulinemia-induced microglial mitochondrial dynamic and metabolic alterations lead to neuroinflammation in vivo and in vitro
Xiaohan Yang, Yuan Xu, Wenting Gao, Li Wang, Xinnan Zhao, Gang Liu, Kai Fan, Shuang Liu, Huimin Hao, Siyan Qu, Renhou Dong, Xiaokai Ma, Jianmei Ma
Frontiers in Neuroscience.2022;[Epub] CrossRef - Integrated Physiology of the Exocrine and Endocrine Compartments in Pancreatic Diseases
Teresa L. Mastracci, Minoti Apte, Laufey T. Amundadottir, Alexandra Alvarsson, Steven Artandi, Melena D. Bellin, Ernesto Bernal-Mizrachi, Alejandro Caicedo, Martha Campbell-Thompson, Zobeida Cruz-Monserrate, Abdelfattah El Ouaamari, Kyle J. Gaulton, Andre
Pancreas.2022; 51(9): 1061. CrossRef - Intestinal Microbiome Modified by Bariatric Surgery Improves Insulin Sensitivity and Correlates with Increased Brown Fat Activity and Energy Expenditure
jitender Yadav, Tao Liang, Tairan Qin, Nayanan N. Nathan, Katherine J.P Schwenger, Lauren Pickel, Li Xie, Helena Lei, Daniel A. Winer, Heather Maughan, Susan J. Robertson, Minna Woo, Wendy Y. W. Lou, Kate Banks, Timothy Jackson, Allan Okrainec, Susy S. Ho
SSRN Electronic Journal .2022;[Epub] CrossRef - Severe hyperglycemia and insulin resistance in patients with SARS-CoV-2 infection: a report of two cases
Alison H. Affinati, Amisha Wallia, Roma Y. Gianchandani
Clinical Diabetes and Endocrinology.2021;[Epub] CrossRef - Promises and pitfalls of beta cell–replacement therapies
Jelena Kolic, James D. Johnson
Nature Metabolism.2021; 3(8): 1036. CrossRef - Hiding unhealthy heart outcomes in a low-fat diet trial: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial finds that postmenopausal women with established coronary heart disease were at increased risk of an adverse outcome if
Timothy David Noakes
Open Heart.2021; 8(2): e001680. CrossRef - On the causal relationships between hyperinsulinaemia, insulin resistance, obesity and dysglycaemia in type 2 diabetes
James D. Johnson
Diabetologia.2021; 64(10): 2138. CrossRef - Diabetes mellitus and cancer: a system of insulin-like growth factors
E. M. Frantsiyants, E. I. Surikova, I. V. Kaplieva, V. A. Bandovkina, I. V. Neskubina, E. A. Sheiko, M. I. Morozova, I. M. Kotieva
Problems of Endocrinology.2021; 67(5): 34. CrossRef
- High adherence to Western dietary pattern increases breast cancer risk (an EPIC-Spain study)
- Type 1 Diabetes
- Non-Insulin Antidiabetes Treatment in Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis
- Xiaoling Cai, Chu Lin, Wenjia Yang, Lin Nie, Linong Ji
- Diabetes Metab J. 2021;45(3):312-325. Published online March 15, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0171

- 6,241 View
- 272 Download
- 5 Web of Science
- 5 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
- In order to evaluate the efficacy and side effects of the non-insulin antidiabetes medications as an adjunct treatment in type 1 diabetes mellitus (T1DM), we conducted systematic searches in MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials for randomized controlled trials published between the date of inception and March 2020 to produce a systematic review and meta-analysis. Overall, 57 studies were included. Compared with placebo, antidiabetes agents in adjunct to insulin treatment resulted in significant reduction in glycosylated hemoglobin (weighted mean difference [WMD], –0.30%; 95% confidence interval [CI], –0.34 to –0.25%; P<0.01) and body weight (WMD, –2.15 kg; 95% CI, –2.77 to –1.53 kg; P<0.01), and required a significantly lower dosage of insulin (WMD, –5.17 unit/day; 95% CI, –6.77 to –3.57 unit/day; P<0.01). Compared with placebo, antidiabetes agents in adjunct to insulin treatment increased the risk of hypoglycemia (relative risk [RR], 1.04; 95% CI, 1.01 to 1.08; P=0.02) and gastrointestinal side effects (RR, 1.99; 95% CI, 1.61 to 2.46; P<0.01) in patients with T1DM. Compared with placebo, the use of non-insulin antidiabetes agents in addition to insulin could lead to glycemic improvement, weight control and lower insulin dosage, while they might be associated with increased risks of hypoglycemia and gastrointestinal side effects in patients with T1DM.
-
Citations
Citations to this article as recorded by- Dioscin: Therapeutic potential for diabetes and complications
Haoyang Gao, Ze Wang, Danlin Zhu, Linlin Zhao, Weihua Xiao
Biomedicine & Pharmacotherapy.2024; 170: 116051. CrossRef - The Impact of Body Mass Index, Residual Beta Cell Function and Estimated Glucose Disposal Rate on the Development of Double Diabetes and Microvascular Complications in Patients With Type 1 Diabetes Mellitus
Rameez Raja Bhagadurshah, Subbiah Eagappan, Raghavan Kasthuri Santharam, Sridhar Subbiah
Cureus.2023;[Epub] CrossRef - Prescribing patterns of adjunctive therapy for the treatment of type 1 diabetes mellitus among Australian endocrinologists
Patrice Forner, Jennifer Snaith, Jerry R. Greenfield
Internal Medicine Journal.2023;[Epub] CrossRef - Type 1 diabetes glycemic management: Insulin therapy, glucose monitoring, and automation
Bruce A. Perkins, Jennifer L. Sherr, Chantal Mathieu
Science.2021; 373(6554): 522. CrossRef - Current Advances of Artificial Pancreas Systems: A Comprehensive Review of the Clinical Evidence
Sun Joon Moon, Inha Jung, Cheol-Young Park
Diabetes & Metabolism Journal.2021; 45(6): 813. CrossRef
- Dioscin: Therapeutic potential for diabetes and complications
- Drug/Regimen
- Lobeglitazone: A Novel Thiazolidinedione for the Management of Type 2 Diabetes Mellitus
- Jaehyun Bae, Taegyun Park, Hyeyoung Kim, Minyoung Lee, Bong-Soo Cha
- Diabetes Metab J. 2021;45(3):326-336. Published online April 19, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0272
- 9,789 View
- 405 Download
- 22 Web of Science
- 23 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
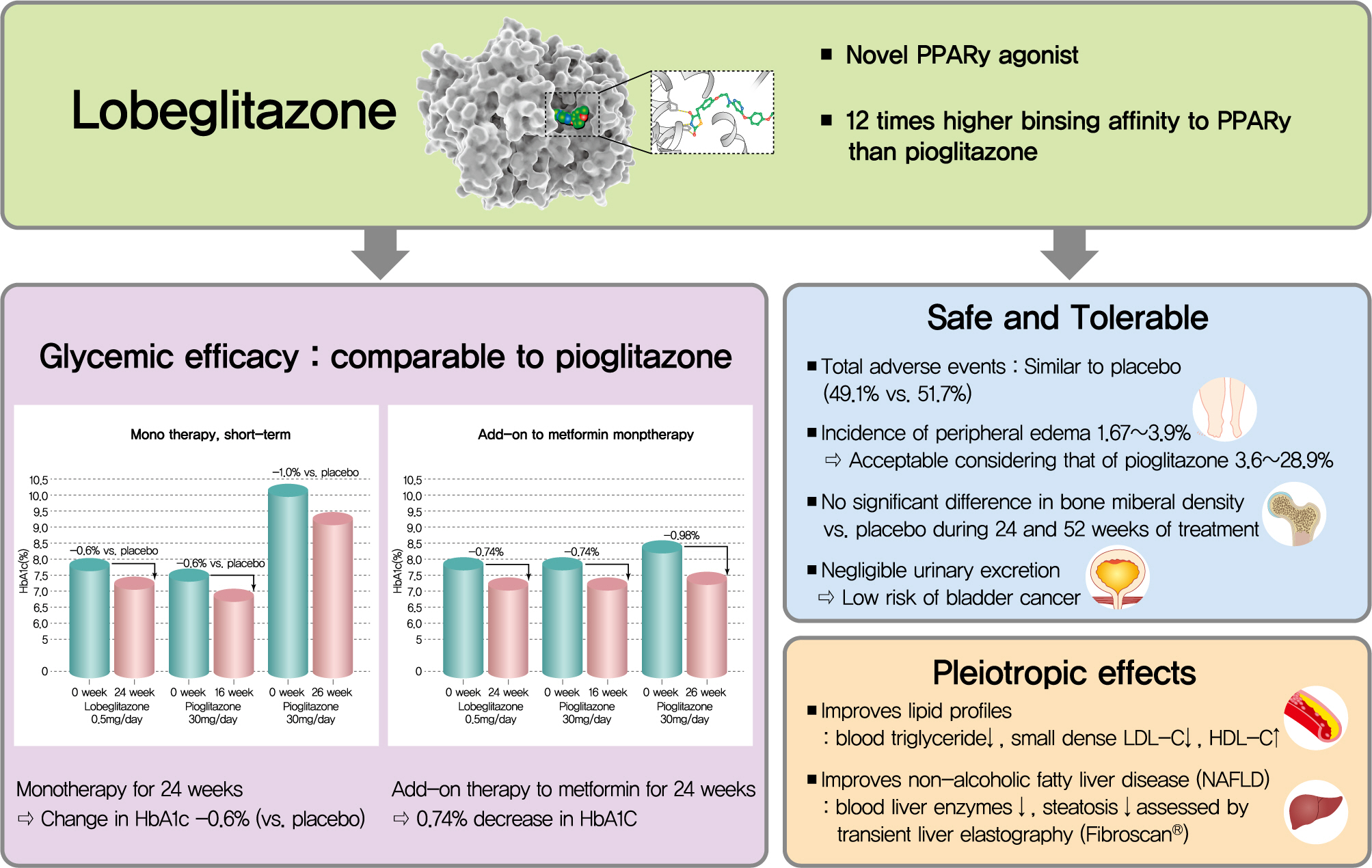
- Type 2 diabetes mellitus (T2DM) is characterized by insulin resistance and β-cell dysfunction. Among available oral antidiabetic agents, only the thiazolidinediones (TZDs) primarily target insulin resistance. TZDs improve insulin sensitivity by activating peroxisome proliferator-activated receptor γ. Rosiglitazone and pioglitazone have been used widely for T2DM treatment due to their potent glycemic efficacy and low risk of hypoglycemia. However, their use has decreased because of side effects and safety issues, such as cardiovascular concerns and bladder cancer. Lobeglitazone (Chong Kun Dang Pharmaceutical Corporation), a novel TZD, was developed to meet the demands for an effective and safe TZD. Lobeglitazone shows similar glycemic efficacy to pioglitazone, with a lower effective dose, and favorable safety results. It also showed pleiotropic effects in preclinical and clinical studies. In this article, we summarize the pharmacologic, pharmacokinetic, and clinical characteristics of lobeglitazone.
-
Citations
Citations to this article as recorded by- Etiology of Drug-Induced Edema: A Review of Dihydropyridine, Thiazolidinedione, and Other Medications Causing Edema
Evan S Sinnathamby, Bretton T Urban, Robert A Clark, Logan T Roberts, Audrey J De Witt, Danielle M Wenger, Aya Mouhaffel, Olga Willett, Shahab Ahmadzadeh, Sahar Shekoohi, Alan D Kaye, Giustino Varrassi
Cureus.2024;[Epub] CrossRef - Novel thiazolidin-4-one benzenesulfonamide hybrids as PPARγ agonists: Design, synthesis and in vivo anti-diabetic evaluation
Islam H. Ali, Rasha M. Hassan, Ahmed M. El Kerdawy, Mahmoud T. Abo-Elfadl, Heba M.I. Abdallah, Francesca Sciandra, Iman A.Y. Ghannam
European Journal of Medicinal Chemistry.2024; 269: 116279. CrossRef - The role of the methoxy group in approved drugs
Debora Chiodi, Yoshihiro Ishihara
European Journal of Medicinal Chemistry.2024; : 116364. CrossRef - Thiazolidinedione an auspicious scaffold as PPAR-γ agonist: its possible mechanism to Manoeuvre against insulin resistant diabetes mellitus
Sourav Basak, Anjali Murmu, Balaji Wamanrao Matore, Partha Pratim Roy, Jagadish Singh
European Journal of Medicinal Chemistry Reports.2024; 11: 100160. CrossRef - Efficacy and Safety of Novel Thiazolidinedione Rivoglitazone in Type-2 Diabetes a Meta-Analysis
Deep Dutta, Jyoti Kadian, Indira Maisnam, Ashok Kumar, Saptarshi Bhattacharya, Meha Sharma
Indian Journal of Endocrinology and Metabolism.2023; 27(4): 286. CrossRef - Efficacy and safety of novel thiazolidinedione lobeglitazone for managing type-2 diabetes a meta-analysis
Deep Dutta, Saptarshi Bhattacharya, Manoj Kumar, Priyankar K. Datta, Ritin Mohindra, Meha Sharma
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(1): 102697. CrossRef - Efficacy and safety of lobeglitazone, a new Thiazolidinedione, as compared to the standard of care in type 2 diabetes mellitus: A systematic review and meta-analysis
Shashank R. Joshi, Saibal Das, Suja Xaviar, Shambo Samrat Samajdar, Indranil Saha, Sougata Sarkar, Shatavisa Mukherjee, Santanu Kumar Tripathi, Jyotirmoy Pal, Nandini Chatterjee
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(1): 102703. CrossRef - Synthesis, Characterization, and Pharmacokinetic Studies of Thiazolidine-2,4-Dione Derivatives
Bushra Ansari, Haroon Khan, Muhammad Saeed Jan, Khalaf F. Alsharif, Khalid J. Alzahrani, Umer Rashid, Abdul Saboor Pirzada, Vinod Kumar Tiwari
Journal of Chemistry.2023; 2023: 1. CrossRef - Will lobeglitazone rival pioglitazone? A systematic review and critical appraisal
Kalyan Kumar Gangopadhyay, Awadhesh Kumar Singh
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(4): 102747. CrossRef - Evaluation of pharmacokinetic interactions between lobeglitazone, empagliflozin, and metformin in healthy subjects
Heeyoung Kim, Choon Ok Kim, Hyeonsoo Park, Min Soo Park, Dasohm Kim, Taegon Hong, Yesong Shin, Byung Hak Jin
Translational and Clinical Pharmacology.2023; 31(1): 59. CrossRef - Lobeglitazone, a novel thiazolidinedione, for secondary prevention in patients with ischemic stroke: a nationwide nested case-control study
Joonsang Yoo, Jimin Jeon, Minyoul Baik, Jinkwon Kim
Cardiovascular Diabetology.2023;[Epub] CrossRef - Complementary effects of dapagliflozin and lobeglitazone on metabolism in a diet-induced obese mouse model
Yun Kyung Lee, Tae Jung Oh, Ji In Lee, Bo Yoon Choi, Hyen Chung Cho, Hak Chul Jang, Sung Hee Choi
European Journal of Pharmacology.2023; 957: 175946. CrossRef - Thiazolidinediones: Recent Development in Analytical Methodologies
Tarang Patel, Vatsal Patel
Journal of Chromatographic Science.2023;[Epub] CrossRef - Current Clinical Trial Status and Future Prospects of PPAR-Targeted Drugs for Treating Nonalcoholic Fatty Liver Disease
Shotaro Kamata, Akihiro Honda, Isao Ishii
Biomolecules.2023; 13(8): 1264. CrossRef - Lobeglitazone inhibits LPS-induced NLRP3 inflammasome activation and inflammation in the liver
Hye-Young Seo, So-Hee Lee, Ji Yeon Park, Eugene Han, Sol Han, Jae Seok Hwang, Mi Kyung Kim, Byoung Kuk Jang, Kenji Fujiwara
PLOS ONE.2023; 18(8): e0290532. CrossRef - Insulin sensitizers in 2023: lessons learned and new avenues for investigation
Jerry R. Colca, Steven P. Tanis, Rolf F. Kletzien, Brian N. Finck
Expert Opinion on Investigational Drugs.2023; 32(9): 803. CrossRef - Treatment of type 2 diabetes mellitus with stem cells and antidiabetic drugs: a dualistic and future-focused approach
Priyamvada Amol Arte, Kanchanlata Tungare, Mustansir Bhori, Renitta Jobby, Jyotirmoi Aich
Human Cell.2023; 37(1): 54. CrossRef - Lobeglitazone and Its Therapeutic Benefits: A Review
Balamurugan M, Sarumathy S, Robinson R
Cureus.2023;[Epub] CrossRef - A double‐blind, Randomized controlled trial on glucose‐lowering EFfects and safety of adding 0.25 or 0.5 mg lobeglitazone in type 2 diabetes patients with INadequate control on metformin and dipeptidyl peptidase‐4 inhibitor therapy: REFIND study
Soree Ryang, Sang Soo Kim, Ji Cheol Bae, Ji Min Han, Su Kyoung Kwon, Young Il Kim, Il Seong Nam‐Goong, Eun Sook Kim, Mi‐kyung Kim, Chang Won Lee, Soyeon Yoo, Gwanpyo Koh, Min Jeong Kwon, Jeong Hyun Park, In Joo Kim
Diabetes, Obesity and Metabolism.2022; 24(9): 1800. CrossRef - Effect of the addition of thiazolidinedione to sodium-glucose cotransporter 2 inhibitor therapy on lipid levels in type 2 diabetes mellitus: a retrospective study using Korean National Health Insurance Service data
Taegyun Park, Kyungdo Han, Dongwook Shin, Jongho Park
Cardiovascular Prevention and Pharmacotherapy.2022; 4(3): 114. CrossRef - Design of Improved Antidiabetic Drugs: A Journey from Single to Multitarget Agents
Vassiliki‐Panagiota Tassopoulou, Ariadni Tzara, Angeliki P. Kourounakis
ChemMedChem.2022;[Epub] CrossRef - A Real-World Study of Long-Term Safety and Efficacy of Lobeglitazone in Korean Patients with Type 2 Diabetes Mellitus
Bo-Yeon Kim, Hyuk-Sang Kwon, Suk Kyeong Kim, Jung-Hyun Noh, Cheol-Young Park, Hyeong-Kyu Park, Kee-Ho Song, Jong Chul Won, Jae Myung Yu, Mi Young Lee, Jae Hyuk Lee, Soo Lim, Sung Wan Chun, In-Kyung Jeong, Choon Hee Chung, Seung Jin Han, Hee-Seok Kim, Ju-Y
Diabetes & Metabolism Journal.2022; 46(6): 855. CrossRef - Lobeglitazone Exerts Anti-Inflammatory Effect in Lipopolysaccharide-Induced Bone-Marrow Derived Macrophages
Dabin Jeong, Wan-Kyu Ko, Seong-Jun Kim, Gong-Ho Han, Daye Lee, Seung-Hun Sheen, Seil Sohn
Biomedicines.2021; 9(10): 1432. CrossRef
- Etiology of Drug-Induced Edema: A Review of Dihydropyridine, Thiazolidinedione, and Other Medications Causing Edema
- Early Glycosylated Hemoglobin Target Achievement Predicts Clinical Outcomes in Patients with Newly Diagnosed Type 2 Diabetes Mellitus
- Joonyub Lee, Jae Hyoung Cho
- Diabetes Metab J. 2021;45(3):337-338. Published online May 25, 2021
- DOI: https://doi.org/10.4093/dmj.2021.0078
- Correction in: Diabetes Metab J 2021;45(4):621
- 4,028 View
- 209 Download
- 4 Web of Science
- 3 Crossref
-
 PDF
PDF PubReader
PubReader  ePub
ePub -
Citations
Citations to this article as recorded by- Evaluation of Left Ventricular Function in Diabetes Patients with Microvascular Disease by Three-Dimensional Speckle Tracking Imaging
青 周
Advances in Clinical Medicine.2023; 13(12): 18908. CrossRef - Association of long-term visit-to-visit variability of HbA1c and fasting glycemia with hypoglycemia in type 2 diabetes mellitus
Chen Long, Yaling Tang, Jiangsheng Huang, Suo Liu, Zhenhua Xing
Frontiers in Endocrinology.2022;[Epub] CrossRef - Time to Reach Target Glycosylated Hemoglobin Is Associated with Long-Term Durable Glycemic Control and Risk of Diabetic Complications in Patients with Newly Diagnosed Type 2 Diabetes Mellitus: A 6-Year Observational Study (Diabetes Metab J 2021;45:368-78)
Kyoung Jin Kim, Jimi Choi, Jae Hyun Bae, Kyeong Jin Kim, Hye Jin Yoo, Ji A Seo, Nan Hee Kim, Kyung Mook Choi, Sei Hyun Baik, Sin Gon Kim, Nam Hoon Kim
Diabetes & Metabolism Journal.2021; 45(4): 617. CrossRef
- Evaluation of Left Ventricular Function in Diabetes Patients with Microvascular Disease by Three-Dimensional Speckle Tracking Imaging
- Drug/Regimen
- Effect of Dapagliflozin as an Add-on Therapy to Insulin on the Glycemic Variability in Subjects with Type 2 Diabetes Mellitus (DIVE): A Multicenter, Placebo-Controlled, Double-Blind, Randomized Study
- Seung-Hwan Lee, Kyung-Wan Min, Byung-Wan Lee, In-Kyung Jeong, Soon-Jib Yoo, Hyuk-Sang Kwon, Yoon-Hee Choi, Kun-Ho Yoon
- Diabetes Metab J. 2021;45(3):339-348. Published online May 28, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0203

- 8,290 View
- 332 Download
- 12 Web of Science
- 15 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
Background Glycemic variability is associated with the development of diabetic complications and hypoglycemia. However, the effect of sodium-glucose transporter 2 (SGLT2) inhibitors on glycemic variability is controversial. We aimed to examine the effect of dapagliflozin as an add-on therapy to insulin on the glycemic variability assessed using continuous glucose monitoring (CGM) in subjects with type 2 diabetes mellitus.
Methods In this multicenter, placebo-controlled, double-blind, randomized study, 84 subjects received 10 mg of dapagliflozin (
n =41) or the placebo (n =43) for 12 weeks. CGM was performed before and after treatment to compare the changes in glycemic variability measures (standard deviation [SD], mean amplitude of glycemic excursions [MAGEs]).Results At week 12, significant reductions in glycosylated hemoglobin (−0.74%±0.66% vs. 0.01%±0.65%,
P <0.001), glycated albumin (−3.94%±2.55% vs. −0.67%±2.48%,P <0.001), and CGM-derived mean glucose (−41.6±39.2 mg/dL vs. 1.1±46.2 mg/dL,P <0.001) levels were observed in the dapagliflozin group compared with the placebo group. SD and MAGE were significantly decreased in the dapagliflozin group, but not in the placebo group. However, the difference in ΔSD and ΔMAGE failed to reach statistical significance between two groups. No significant differences in the incidence of safety endpoints were observed between the two groups.Conclusion Dapagliflozin effectively decreased glucose levels, but not glucose variability, after 12 weeks of treatment in participants with type 2 diabetes mellitus receiving insulin treatment. The role of SGLT2 inhibitors in glycemic variability warrants further investigations.
-
Citations
Citations to this article as recorded by- Selective sodium-glucose cotransporter-2 inhibitors in the improvement of hemoglobin and hematocrit in patients with type 2 diabetes mellitus: a network meta-analysis
Yuanyuan Luo, Ruojing Bai, Wei Zhang, Guijun Qin
Frontiers in Endocrinology.2024;[Epub] CrossRef - Continuous Glucose Monitoring Profiles and Health Outcomes After Dapagliflozin Plus Saxagliptin vs Insulin Glargine
Donald C Simonson, Marcia A Testa, Ella Ekholm, Maxwell Su, Tina Vilsbøll, Serge A Jabbour, Marcus Lind
The Journal of Clinical Endocrinology & Metabolism.2024;[Epub] CrossRef - Impact of empagliflozin on insulin needs in patients with heart failure and diabetes: An EMPEROR‐Pooled analysis
Khawaja M. Talha, Jennifer Green, Gerasimos Filippatos, Stuart Pocock, Faiez Zannad, Martina Brueckmann, Elke Schueler, Anne Pernille Ofstad, João Pedro Ferreira, Stefan D. Anker, Javed Butler, Julio Rosenstock, Milton Packer
Diabetes, Obesity and Metabolism.2024;[Epub] CrossRef - Risk of Urinary Tract Infection in Patients with Type 2 Diabetes Mellitus Treated with Dapagliflozin: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Zhigui Zheng, Dongyuan He, Jianguo Chen, Xiaohui Xie, Yunan Lu, Binbin Wu, Xinxin Jiang
Clinical Drug Investigation.2023; 43(4): 209. CrossRef - Effect of SGLT2 Inhibitors and Metformin on Inflammatory and Prognostic
Biomarkers in Type 2 Diabetes Patients
Yang Cao, Ning Liang, Ting Liu, Jingai Fang, Xiaodong Zhang
Endocrine, Metabolic & Immune Disorders - Drug Targets.2023; 23(4): 530. CrossRef - What is Glycaemic Variability and which Pharmacological Treatment Options are Effective? A Narrative Review
Juan Miguel Huertas Cañas, Maria Alejandra Gomez Gutierrez, Andres Bedoya Ossa
European Endocrinology.2023; 19(2): 4. CrossRef - La variabilité glycémique : un facteur de risque singulier à conjuguer au pluriel
Louis Monnier, Claude Colette, Fabrice Bonnet, David Owens
Médecine des Maladies Métaboliques.2022; 16(1): 15. CrossRef - Association between Variability of Metabolic Risk Factors and Cardiometabolic Outcomes
Min Jeong Park, Kyung Mook Choi
Diabetes & Metabolism Journal.2022; 46(1): 49. CrossRef - Effect of sodium-glucose cotransporter-2 (SGLT2) inhibitors on serum urate levels in patients with and without diabetes: a systematic review and meta-regression of 43 randomized controlled trials
Alicia Swee Yan Yip, Shariel Leong, Yao Hao Teo, Yao Neng Teo, Nicholas L. X. Syn, Ray Meng See, Caitlin Fern Wee, Elliot Yeung Chong, Chi-Hang Lee, Mark Y. Chan, Tiong-Cheng Yeo, Raymond C. C. Wong, Ping Chai, Ching-Hui Sia
Therapeutic Advances in Chronic Disease.2022; 13: 204062232210835. CrossRef - Hypoglycemic agents and glycemic variability in individuals with type 2 diabetes: A systematic review and network meta-analysis
SuA Oh, Sujata Purja, Hocheol Shin, Minji Kim, Eunyoung Kim
Diabetes and Vascular Disease Research.2022; 19(3): 147916412211068. CrossRef - The Clinical Effect of Dapagliflozin in Patients with Angiographically Confirmed Coronary Artery Disease and Concomitant Type 2 Diabetes Mellitus
Yana Yu. Dzhun, Yevhen Yu. Marushko, Yanina A. Saienko, Nadiya M. Rudenko, Borys M. Mankovsky
Ukrainian Journal of Cardiovascular Surgery.2022; 30(3): 35. CrossRef - Stress-Induced Hyperglycaemia in Non-Diabetic Patients with Acute Coronary Syndrome: From Molecular Mechanisms to New Therapeutic Perspectives
Alessandro Bellis, Ciro Mauro, Emanuele Barbato, Antonio Ceriello, Antonio Cittadini, Carmine Morisco
International Journal of Molecular Sciences.2021; 22(2): 775. CrossRef - Glycemic Variability Impacted by SGLT2 Inhibitors and GLP 1 Agonists in Patients with Diabetes Mellitus: A Systematic Review and Meta-Analysis
Heeyoung Lee, Se-eun Park, Eun-Young Kim
Journal of Clinical Medicine.2021; 10(18): 4078. CrossRef - Effect of Dapagliflozin on Glycemic Variability in Patients with Type 2 Diabetes under Insulin Glargine Combined with Other Oral Hypoglycemic Drugs
Menghui Luo, Xiaocen Kong, Huiying Wang, Xiaofang Zhai, Tingting Cai, Bo Ding, Yun Hu, Ting Jing, Xiaofei Su, Huiqin Li, Jianhua Ma, Yoshifumi Saisho
Journal of Diabetes Research.2020; 2020: 1. CrossRef - Time in Range from Continuous Glucose Monitoring: A Novel Metric for Glycemic Control
Jee Hee Yoo, Jae Hyeon Kim
Diabetes & Metabolism Journal.2020; 44(6): 828. CrossRef
- Selective sodium-glucose cotransporter-2 inhibitors in the improvement of hemoglobin and hematocrit in patients with type 2 diabetes mellitus: a network meta-analysis
- Complications
- Association of Urinary N-Acetyl-β-D-Glucosaminidase with Cardiovascular Autonomic Neuropathy in Type 1 Diabetes Mellitus without Nephropathy
- Min Sun Choi, Ji Eun Jun, Sung Woon Park, Jee Hee Yoo, Jiyeon Ahn, Gyuri Kim, Sang-Man Jin, Kyu Yeon Hur, Moon-Kyu Lee, Jae Hyeon Kim
- Diabetes Metab J. 2021;45(3):349-357. Published online February 2, 2021
- DOI: https://doi.org/10.4093/dmj.2019.0211
- 5,638 View
- 121 Download
- 1 Web of Science
- 2 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub 
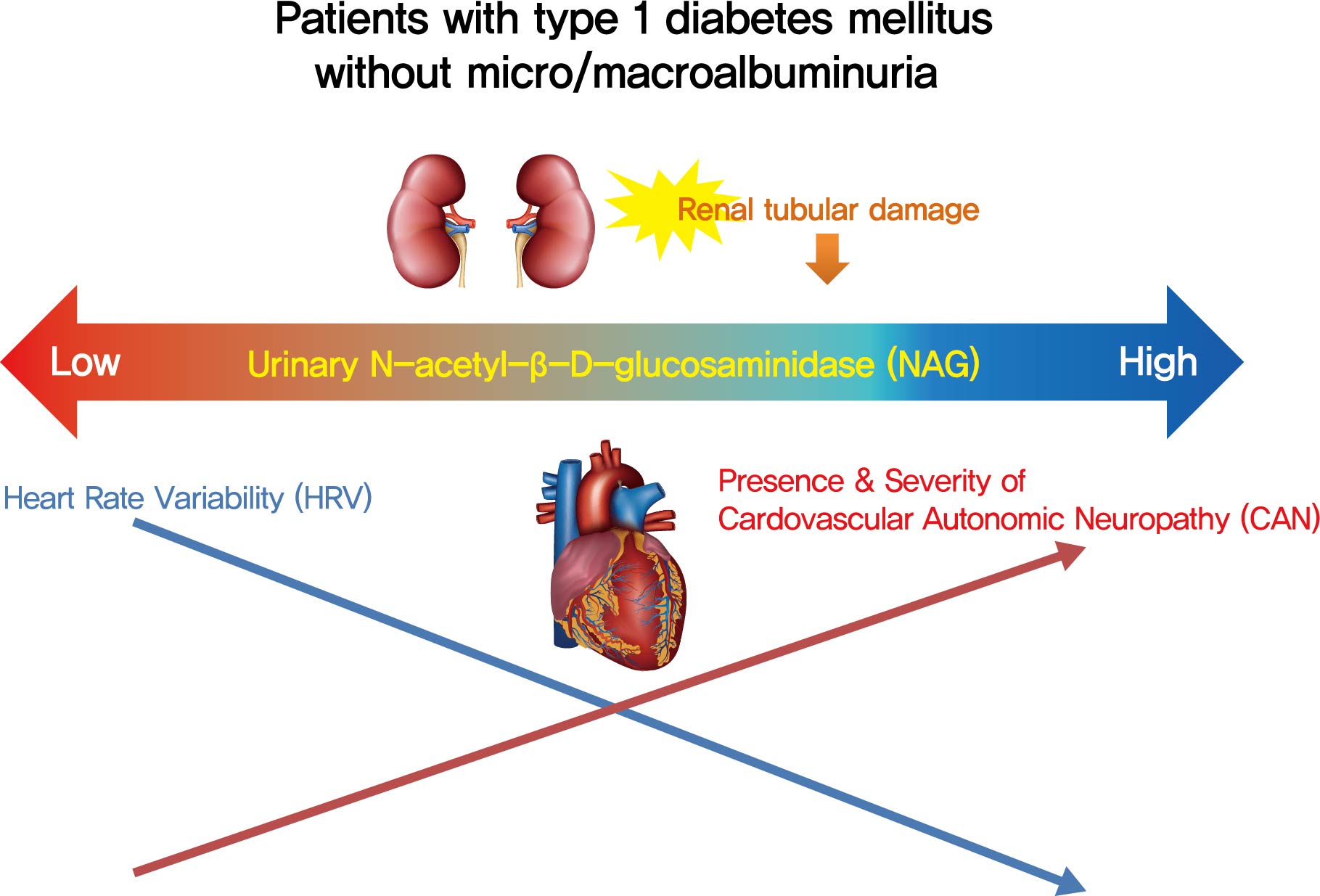
- Background
Cardiovascular autonomic neuropathy (CAN) is a common microvascular complication of diabetes and related to albuminuria in diabetic nephropathy (DN). Urinary N-acetyl-β-D-glucosaminidase (uNAG) is a renal tubular injury marker which has been reported as an early marker of DN even in patients with normoalbuminuria. This study evaluated whether uNAG is associated with the presence and severity of CAN in patients with type 1 diabetes mellitus (T1DM) without nephropathy.
Methods
This cross-sectional study comprised 247 subjects with T1DM without chronic kidney disease and albuminuria who had results for both uNAG and autonomic function tests within 3 months. The presence of CAN was assessed by age-dependent reference values for four autonomic function tests. Total CAN score was assessed as the sum of the partial points of five cardiovascular reflex tests and was used to estimatethe severity of CAN. The correlations between uNAG and heart rate variability (HRV) parameters were analyzed.
Results
The association between log-uNAG and presence of CAN was significant in a multivariate logistic regression model (adjusted odds ratio, 2.39; 95% confidence interval [CI], 1.08 to 5.28; P=0.031). Total CAN score was positively associated with loguNAG (β=0.261, P=0.026) in the multivariate linear regression model. Log-uNAG was inversely correlated with frequency-domain and time-domain indices of HRV.
Conclusion
This study verified the association of uNAG with presence and severity of CAN and changes in HRV in T1DM patients without nephropathy. The potential role of uNAG should be further assessed for high-risk patients for CAN in T1DM patients without nephropathy. -
Citations
Citations to this article as recorded by- Determination of Diabetes-associated Cardiovascular Autonomic Neuropathy Risk Factors among Insulin and Non-insulin Dependent Diabetics
Ibrahim Abdulsada, Zain Alabdeen Obaid, Farah Almerza, Mays Alwaeli, Anmar Al-Elayawi, Taha Al-Dayyeni, Harir Al-Tuhafy
The Journal of Medical Research.2023; 9(6): 141. CrossRef - Association between carotid atherosclerosis and presence of intracranial atherosclerosis using three-dimensional high-resolution vessel wall magnetic resonance imaging in asymptomatic patients with type 2 diabetes
Ji Eun Jun, You-Cheol Hwang, Kyu Jeong Ahn, Ho Yeon Chung, Geon-Ho Jahng, Soonchan Park, In-Kyung Jeong, Chang-Woo Ryu
Diabetes Research and Clinical Practice.2022; 191: 110067. CrossRef
- Determination of Diabetes-associated Cardiovascular Autonomic Neuropathy Risk Factors among Insulin and Non-insulin Dependent Diabetics
- Complications
- Association between Sleep Quality and Painless Diabetic Peripheral Neuropathy Assessed by Current Perception Threshold in Type 2 Diabetes Mellitus
- Dughyun Choi, Bo-Yeon Kim, Chan-Hee Jung, Chul-Hee Kim, Ji-Oh Mok
- Diabetes Metab J. 2021;45(3):358-367. Published online August 6, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0219
- 5,947 View
- 155 Download
- 1 Web of Science
- 3 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
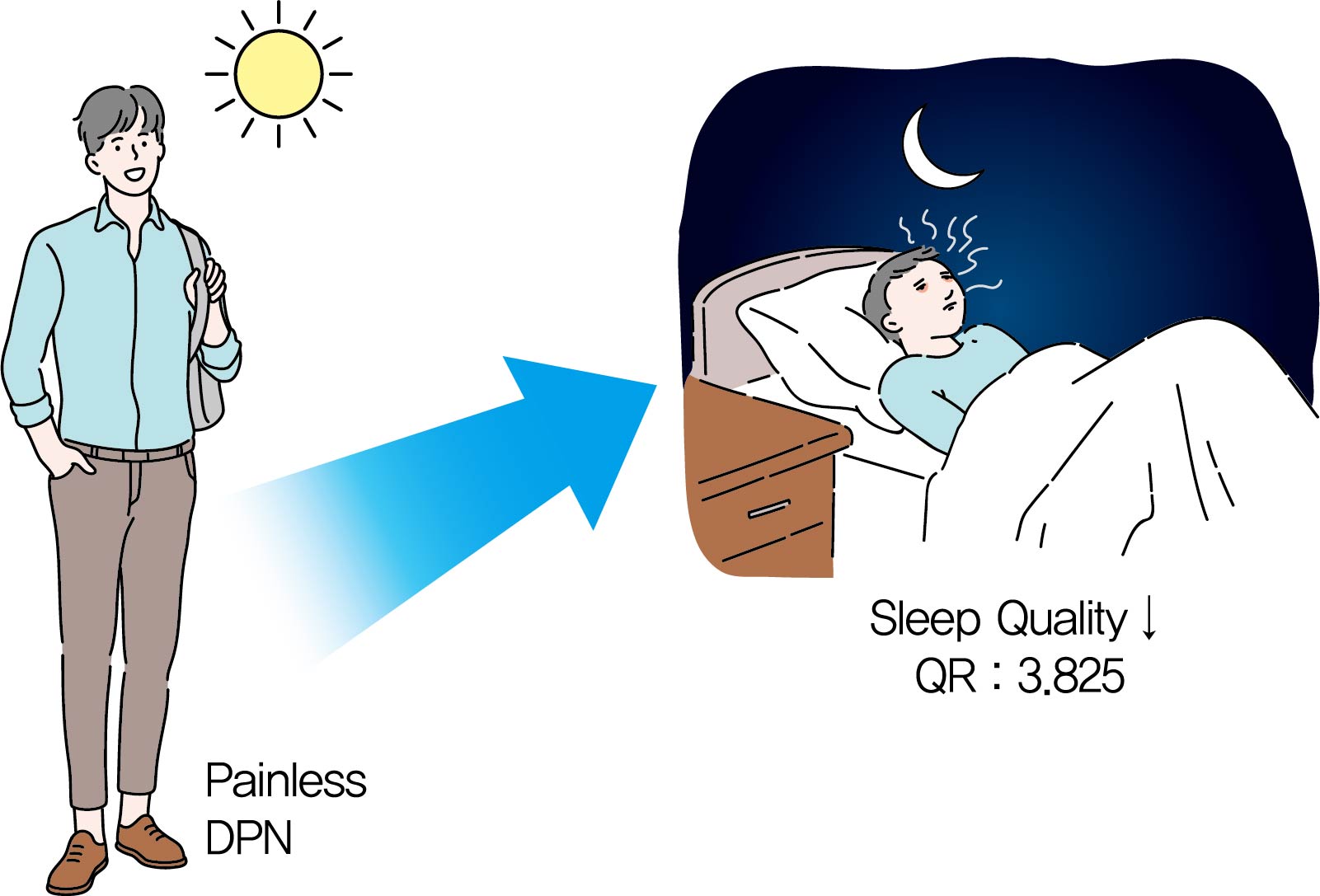
Background It is known that the painful sensation of diabetic peripheral neuropathy (DPN) results in sleep problems in type 2 diabetes mellitus (T2DM). However, it is not known that the painless DPN also is associated with poor sleep quality in T2DM. The purpose of the current study was to investigate the association between painless DPN and poor sleep quality in T2DM.
Methods A total of 146 patients of T2DM who do not have any painful symptoms of DPN were recruited into the study. Among the patients, painless DPN was diagnosed by using the current perception threshold test. Sleep quality was assessed using the Pittsburgh Sleep Quality Index questionnaire.
Results The percentage of painless DPN was significantly higher in the poor sleep quality group than the good sleep quality group (70.0% vs. 35.5%,
P <0.001). In the subscale results, stimulus values at 2,000 Hz, hypoesthesia and hyperesthesia were more common in the poor sleep quality group than in the good sleep quality group (45.7% vs. 25.0%,P =0.009; 34.3% vs. 18.4%,P =0.029; 40.0% vs. 19.7%,P =0.007, respectively). The association of painless DPN and poor sleep quality remained significant after adjustment for significant covariates (odds ratio, 3.825; 95% confidence interval, 1.674 to 8.742;P <0.001).Conclusion The current study showed that painless DPN was associated with poor sleep quality. Future studies are required to clarify the pathophysiologic causal relationship between painless DPN and sleep quality.
-
Citations
Citations to this article as recorded by- Deteriorated sleep quality and associate factors in patients with type 2 diabetes mellitus complicated with diabetic peripheral neuropathy
Lin Fu, Liping Zhong, Xin Liao, Lingrui Wang, Youyi Wang, Xiuquan Shi, Yanna Zhou
PeerJ.2024; 12: e16789. CrossRef - Sleep impairment: Is it an overlooked burden in painful diabetic peripheral neuropathy? A single-centre, cross-sectional study from south India
Adlin Lawrence, Himsikhar Khataniar, Sinimol Joseph, Thenmozhi Nagarajan, Soumya Umesh, John Michael Raj A
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2022; 16(8): 102568. CrossRef - Sleep: an emerging therapeutic target in diabetes care
Nishant Raizada, S. V. Madhu
International Journal of Diabetes in Developing Countries.2021; 41(1): 1. CrossRef
- Deteriorated sleep quality and associate factors in patients with type 2 diabetes mellitus complicated with diabetic peripheral neuropathy
- Complications
-
- Time to Reach Target Glycosylated Hemoglobin Is Associated with Long-Term Durable Glycemic Control and Risk of Diabetic Complications in Patients with Newly Diagnosed Type 2 Diabetes Mellitus: A 6-Year Observational Study
- Kyoung Jin Kim, Jimi Choi, Jae Hyun Bae, Kyeong Jin Kim, Hye Jin Yoo, Ji A Seo, Nan Hee Kim, Kyung Mook Choi, Sei Hyun Baik, Sin Gon Kim, Nam Hoon Kim
- Diabetes Metab J. 2021;45(3):368-378. Published online October 20, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0046

- 9,387 View
- 344 Download
- 19 Web of Science
- 19 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
- Background
To evaluate the association of time to reach the target glycosylated hemoglobin (HbA1c) level with long-term durable glycemic control and risk of diabetic complications in patients with newly diagnosed type 2 diabetes mellitus (T2DM).
Methods
In a longitudinal observational cohort, 194 patients with T2DM newly diagnosed between January 2011 and March 2013 were followed up over 6 years. Patients were classified according to the time needed to reach the target HbA1c (<7.0%): <3, 3 to 6 (early achievement group), and ≥6 months (late achievement group). Risks of microvascular complications including diabetic retinopathy, nephropathy, and neuropathy as well as macrovascular events including ischemic heart disease, ischemic stroke, and peripheral arterial disease were assessed by multivariable Cox proportional hazards analysis.
Results
During a median follow-up of 6.53 years, 66 microvascular and 14 macrovascular events occurred. Maintenance of durable glycemic control over 6 years was more likely in the early achievement groups than in the late achievement group (34.5%, 30.0%, and 16.1% in <3, 3 to 6, and ≥6 months, respectively, P=0.039). Early target HbA1c achievement was associated with lower risk of composite diabetic complications (adjusted hazard ratio [HR, 0.47; 95% confidence interval [CI], 0.26 to 0.86 in <3 months group) (adjusted HR, 0.50; 95% CI, 0.23 to 1.10 in 3 to 6 months group, in reference to ≥6 months group). Similar trends were maintained for risks of microvascular and macrovascular complications, although statistical significance was not reached for macrovascular complications.
Conclusion
Early target HbA1c achievement was associated with long-term durable glycemic control and reduced risk of diabetic complications in newly diagnosed T2DM. -
Citations
Citations to this article as recorded by- HbA1c As Diabetes Mellitus Biomarker and Its Methods Evolution
Liong Boy Kurniawan
INDONESIAN JOURNAL OF CLINICAL PATHOLOGY AND MEDICAL LABORATORY.2024; 30(2): 191. CrossRef - Efficacy and safety of enavogliflozin vs. dapagliflozin as add-on therapy in patients with type 2 diabetes mellitus based on renal function: a pooled analysis of two randomized controlled trials
Young Sang Lyu, Sangmo Hong, Si Eun Lee, Bo Young Cho, Cheol-Young Park
Cardiovascular Diabetology.2024;[Epub] CrossRef - The effect of health quotient and time management skills on self-management behavior and glycemic control among individuals with type 2 diabetes mellitus
Mengjie Chen, Man Liu, Ying Pu, Juan Wu, Mingjiao Zhang, Hongxia Tang, Laixi Kong, Maoting Guo, Kexue Zhu, Yuxiu Xie, Zhe Li, Bei Deng, Zhenzhen Xiong
Frontiers in Public Health.2024;[Epub] CrossRef - Glycemic control and cardiovascular complications of type 2 diabetes mellitus
I. V. Druk, S. S. Safronova
Meditsinskiy sovet = Medical Council.2023; (13): 130. CrossRef - Effect of viscous soluble dietary fiber on glucose and lipid metabolism in patients with type 2 diabetes mellitus: a systematic review and meta-analysis on randomized clinical trials
Kun Lu, Tingqing Yu, Xinyi Cao, Hui Xia, Shaokang Wang, Guiju Sun, Liang Chen, Wang Liao
Frontiers in Nutrition.2023;[Epub] CrossRef - Construction and validation of a clinical prediction model for asymptomatic obstructive coronary stenosis in patients with carotid stenosis
Cuijie Qin, Chuang Li, Yunpeng Luo, Zhen Li, Hui Cao
Frontiers in Cardiovascular Medicine.2023;[Epub] CrossRef - Risk assessment of rectal anastomotic leakage (RAREAL) after DIXON in non-emergency patients with rectal cancer
Xue-Cong Zheng, Jin-Bo Su, Jin-Jie Zheng
BMC Gastroenterology.2023;[Epub] CrossRef - Evaluation of Left Ventricular Function in Diabetes Patients with Microvascular Disease by Three-Dimensional Speckle Tracking Imaging
青 周
Advances in Clinical Medicine.2023; 13(12): 18908. CrossRef - Validity of the diagnosis of diabetic microvascular complications in Korean national health insurance claim data
Hyung Jun Kim, Moo-Seok Park, Jee-Eun Kim, Tae-Jin Song
Annals of Clinical Neurophysiology.2022; 24(1): 7. CrossRef - Metformin plus a low hypoglycemic risk antidiabetic drug vs. metformin monotherapy for untreated type 2 diabetes mellitus: A meta-analysis of randomized controlled trials
Wei-Tse Hung, Yuan-Jung Chen, Chun-Yu Cheng, Bruce Ovbiagele, Meng Lee, Chia-Yu Hsu
Diabetes Research and Clinical Practice.2022; 189: 109937. CrossRef - Peripheral arterial disease progression and ankle brachial index: a cohort study with newly diagnosed patients with type 2 diabetes
João Soares Felício, Franciane Trindade Cunha de Melo, Giovana Miranda Vieira, Vitória Teixeira de Aquino, Fernanda de Souza Parente, Wanderson Maia da Silva, Nivin Mazen Said, Emanuele Rocha da Silva, Ana Carolina Contente Braga de Souza, Maria Clara Ner
BMC Cardiovascular Disorders.2022;[Epub] CrossRef - Association of long-term visit-to-visit variability of HbA1c and fasting glycemia with hypoglycemia in type 2 diabetes mellitus
Chen Long, Yaling Tang, Jiangsheng Huang, Suo Liu, Zhenhua Xing
Frontiers in Endocrinology.2022;[Epub] CrossRef - The Degree of Glycemic Control for the First Three Months Determines the Next Seven Years
Nami Lee, Dae Jung Kim
Journal of Korean Medical Science.2022;[Epub] CrossRef - Inhibition of advanced glycation end products and protein oxidation by leaf extracts and phenolics from Chilean bean landraces
Felipe Ávila, Nadia Cruz, Jazmin Alarcon-Espósito, Nélida Nina, Hernán Paillan, Katherine Márquez, Denis Fuentealba, Alberto Burgos-Edwards, Cristina Theoduloz, Carmina Vejar-Vivar, Guillermo Schmeda-Hirschmann
Journal of Functional Foods.2022; 98: 105270. CrossRef - Mediation Effect of Self-Efficacy Between Health Beliefs and Glycated Haemoglobin Levels in Elderly Patients with Type 2 Diabetes Mellitus: A Cross-Sectional Study
Anqi Zhang, Jinsong Wang, Xiaojuan Wan, Jing Zhang, Zihe Guo, Yamin Miao, Shuhan Zhao, Shuo Bai, Ziyi Zhang, Weiwei Yang
Patient Preference and Adherence.2022; Volume 16: 3015. CrossRef - Early Glycosylated Hemoglobin Target Achievement Predicts Clinical Outcomes in Patients with Newly Diagnosed Type 2 Diabetes Mellitus
Joonyub Lee, Jae Hyoung Cho
Diabetes & Metabolism Journal.2021; 45(3): 337. CrossRef - Time to Reach Target Glycosylated Hemoglobin Is Associated with Long-Term Durable Glycemic Control and Risk of Diabetic Complications in Patients with Newly Diagnosed Type 2 Diabetes Mellitus: A 6-Year Observational Study (Diabetes Metab J 2021;45:368-78)
Ja Young Jeon
Diabetes & Metabolism Journal.2021; 45(4): 613. CrossRef - Time to Reach Target Glycosylated Hemoglobin Is Associated with Long-Term Durable Glycemic Control and Risk of Diabetic Complications in Patients with Newly Diagnosed Type 2 Diabetes Mellitus: A 6-Year Observational Study (Diabetes Metab J 2021;45:368-78)
Kyoung Jin Kim, Jimi Choi, Jae Hyun Bae, Kyeong Jin Kim, Hye Jin Yoo, Ji A Seo, Nan Hee Kim, Kyung Mook Choi, Sei Hyun Baik, Sin Gon Kim, Nam Hoon Kim
Diabetes & Metabolism Journal.2021; 45(4): 617. CrossRef - Plasma Nesfatin-1: Potential Predictor and Diagnostic Biomarker for Cognitive Dysfunction in T2DM Patient
Dandan Xu, Yue Yu, Yayun Xu, Jinfang Ge
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2021; Volume 14: 3555. CrossRef
- HbA1c As Diabetes Mellitus Biomarker and Its Methods Evolution
- Cardiovascular risk/Epidemiology
- Increased Risk of Cardiovascular Disease and Mortality in Patients with Diabetes and Coexisting Depression: A Nationwide Population-Based Cohort Study
- Inha Jung, Hyemi Kwon, Se Eun Park, Kyung-Do Han, Yong-Gyu Park, Yang-Hyun Kim, Eun-Jung Rhee, Won-Young Lee
- Diabetes Metab J. 2021;45(3):379-389. Published online December 11, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0008
- 7,504 View
- 235 Download
- 22 Web of Science
- 21 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
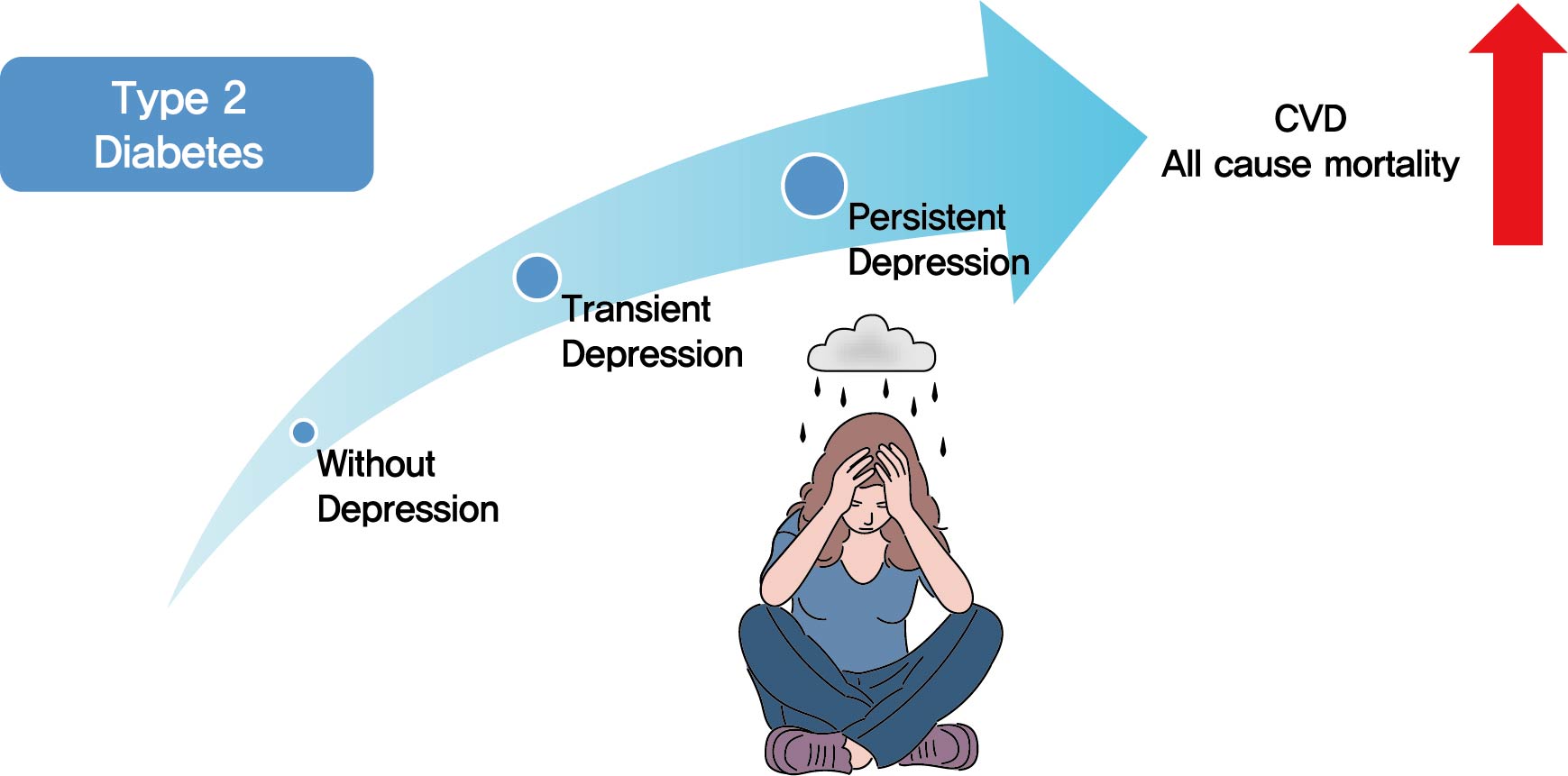
- Background
Previous studies have suggested that depression in patients with diabetes is associated with worse health outcomes. The aim of this study was to evaluate the risk of cardiovascular disease (CVD) and mortality in patients with diabetes with comorbid depression.
Methods
We examined the general health check-up data and claim database of the Korean National Health Insurance Service (NHIS) of 2,668,615 participants with type 2 diabetes mellitus who had examinations between 2009 and 2012. As NHIS database has been established since 2002, those who had been diagnosed with depression or CVD since 2002 were excluded. The 2,228,443 participants were classified into three groups according to the claim history of depression; normal group (n=2,166,979), transient depression group (one episode of depression, n=42,124) and persistent depression group (at least two episodes of depression, n=19,340). The development of CVD and mortality were analyzed from 2009 to 2017.
Results
Those with depression showed a significantly increased risk for stroke (transient depression group: hazard ratio [HR], 1.20; 95% confidence interval [CI], 1.15 to 1.26) (persistent depression group: HR, 1.54; 95% CI, 1.46 to 1.63). Those with depression had an increased risk for myocardial infarction (transient depression group: HR, 1.25; 95% CI, 1.18 to 1.31) (persistent depression group: HR, 1.38; 95% CI, 1.29 to 1.49). The persistent depression group had an increased risk for all-cause mortality (HR, 1.66; 95% CI, 1.60 to 1.72).
Conclusion
Coexisting depression in patients with diabetes has a deleterious effect on the development of CVD and mortality. We suggest that more attention should be given to patients with diabetes who present with depressive symptoms. -
Citations
Citations to this article as recorded by- Psychological resilience mediates the relationship between diabetes distress and depression among persons with diabetes in a multi-group analysis
Ajele Kenni Wojujutari, Erhabor Sunday Idemudia, Lawrence Ejike Ugwu
Scientific Reports.2024;[Epub] CrossRef - The mediating effect of depression on new-onset stroke in diabetic population: Evidence from the China health and retirement longitudinal study
Gege Jiang, Yaoling Wang, Liping Wang, Minfang Chen, Wei Li
Journal of Affective Disorders.2023; 321: 208. CrossRef - Frailty and outcomes in lacunar stroke
Sima Vazquez, Zehavya Stadlan, Justin M Lapow, Eric Feldstein, Smit Shah, Ankita Das, Alexandria F Naftchi, Eris Spirollari, Akash Thaker, Syed Faraz Kazim, Jose F Dominguez, Neisha Patel, Christeena Kurian, Ji Chong, Stephan A Mayer, Gurmeen Kaur, Chirag
Journal of Stroke and Cerebrovascular Diseases.2023; 32(2): 106942. CrossRef - Comparison of Operational Definition of Type 2 Diabetes Mellitus Based on Data from Korean National Health Insurance Service and Korea National Health and Nutrition Examination Survey
Jong Ha Baek, Yong-Moon Park, Kyung Do Han, Min Kyong Moon, Jong Han Choi, Seung-Hyun Ko
Diabetes & Metabolism Journal.2023; 47(2): 201. CrossRef - The Association between Dietary Carotenoid Intake and Risk of Depression among Patients with Cardiometabolic Disease
Jie Liang, Yuhao Wang, Min Chen
International Heart Journal.2023; 64(2): 223. CrossRef - Associations of concomitant retinopathy and depression with mortality in a nationally representative population
Zheng Lyu, Yilin Chen, Zhuoting Zhu, Xiaoyang Luo, Ying Cui, Jie Xie, Zhifan Chen, Junbin Liu, Xiyu Wu, Gabrella Bulloch, Qianli Meng
Journal of Affective Disorders.2023; 336: 15. CrossRef - Clinical insights into the cross-link between mood disorders and type 2 diabetes: A review of longitudinal studies and Mendelian randomisation analyses
Chiara Possidente, Giuseppe Fanelli, Alessandro Serretti, Chiara Fabbri
Neuroscience & Biobehavioral Reviews.2023; 152: 105298. CrossRef - Prevalence of depression and association with all-cause and cardiovascular mortality among individuals with type 2 diabetes: a cohort study based on NHANES 2005–2018 data
Zhen Feng, Wai Kei Tong, Xinyue Zhang, Zhijia Tang
BMC Psychiatry.2023;[Epub] CrossRef - Cholecystectomy Increases the Risk of Type 2 Diabetes in the Korean Population
Ji Hye Huh, Kyong Joo Lee, Yun Kyung Cho, Shinje Moon, Yoon Jung Kim, Eun Roh, Kyung-do Han, Dong Hee Koh, Jun Goo Kang, Seong Jin Lee, Sung-Hee Ihm
Annals of Surgery.2023; 278(2): e264. CrossRef - Risk of depression in patients with acromegaly in Korea (2006-2016): a nationwide population-based study
Shinje Moon, Sangmo Hong, Kyungdo Han, Cheol-Young Park
European Journal of Endocrinology.2023; 189(3): 363. CrossRef - The association between cardiovascular drugs and depression/anxiety in patients with cardiovascular disease: A meta-analysis
Lijun Zhang, Yanping Bao, Shuhui Tao, Yimiao Zhao, Meiyan Liu
Pharmacological Research.2022; 175: 106024. CrossRef - Association of mental health with the risk of coronary artery disease in patients with diabetes: A mendelian randomization study
Teng Hu, Fangkun Yang, Kewan He, Jiajun Ying, Hanbin Cui
Nutrition, Metabolism and Cardiovascular Diseases.2022; 32(3): 703. CrossRef - Comorbidity of Type 2 Diabetes Mellitus and Depression: Clinical Evidence and Rationale for the Exacerbation of Cardiovascular Disease
Mengmeng Zhu, Yiwen Li, Binyu Luo, Jing Cui, Yanfei Liu, Yue Liu
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Understanding and Utilizing Claim Data from the Korean National Health Insurance Service (NHIS) and Health Insurance Review & Assessment (HIRA) Database for Research
Dae-Sung Kyoung, Hun-Sung Kim
Journal of Lipid and Atherosclerosis.2022; 11(2): 103. CrossRef - Evaluating Triglyceride and Glucose Index as a Simple and Easy-to-Calculate Marker for All-Cause and Cardiovascular Mortality
Kyung-Soo Kim, Sangmo Hong, You-Cheol Hwang, Hong-Yup Ahn, Cheol-Young Park
Journal of General Internal Medicine.2022; 37(16): 4153. CrossRef - Evaluation of rosmarinic acid against myocardial infarction in maternally separated rats
Himanshu Verma, Anindita Bhattacharjee, Naveen Shivavedi, Prasanta Kumar Nayak
Naunyn-Schmiedeberg's Archives of Pharmacology.2022; 395(10): 1189. CrossRef - Lipid cutoffs for increased cardiovascular disease risk in non-diabetic young people
Mee Kyoung Kim, Kyungdo Han, Hun-Sung Kim, Kun-Ho Yoon, Seung-Hwan Lee
European Journal of Preventive Cardiology.2022; 29(14): 1866. CrossRef - Risk factors associated with mortality among individuals with type 2 diabetes and depression across two cohorts
Christopher Rohde, Jens Steen Nielsen, Jakob Schöllhammer Knudsen, Reimar Wernich Thomsen, Søren Dinesen Østergaard
European Journal of Endocrinology.2022; 187(4): 567. CrossRef - Increased Risk of Cardiovascular Disease and Mortality in Patients with Diabetes and Coexisting Depression: A Nationwide Population-Based Cohort Study (Diabetes Metab J 2021;45:379-89)
Jin Hwa Kim
Diabetes & Metabolism Journal.2021; 45(5): 789. CrossRef - Increased Risk of Cardiovascular Disease and Mortality in Patients with Diabetes and Coexisting Depression: A Nationwide Population-Based Cohort Study (Diabetes Metab J 2021;45:379-89)
Inha Jung, Eun-Jung Rhee, Won-Young Lee
Diabetes & Metabolism Journal.2021; 45(5): 793. CrossRef - Affective Temperament and Glycemic Control – The Psychological Aspect of Obesity and Diabetes Mellitus
Natalia Lesiewska, Anna Kamińska, Roman Junik, Magdalena Michalewicz, Bartłomiej Myszkowski, Alina Borkowska, Maciej Bieliński
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2021; Volume 14: 4981. CrossRef
- Psychological resilience mediates the relationship between diabetes distress and depression among persons with diabetes in a multi-group analysis
- Lifestyle
- Reducing Carbohydrate from Individual Sources Has Differential Effects on Glycosylated Hemoglobin in Type 2 Diabetes Mellitus Patients on Moderate Low-Carbohydrate Diets
- Hajime Haimoto, Shiho Watanabe, Keiko Maeda, Takashi Murase, Kenji Wakai
- Diabetes Metab J. 2021;45(3):390-403. Published online July 21, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0033
- 5,794 View
- 160 Download
- 3 Web of Science
- 3 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
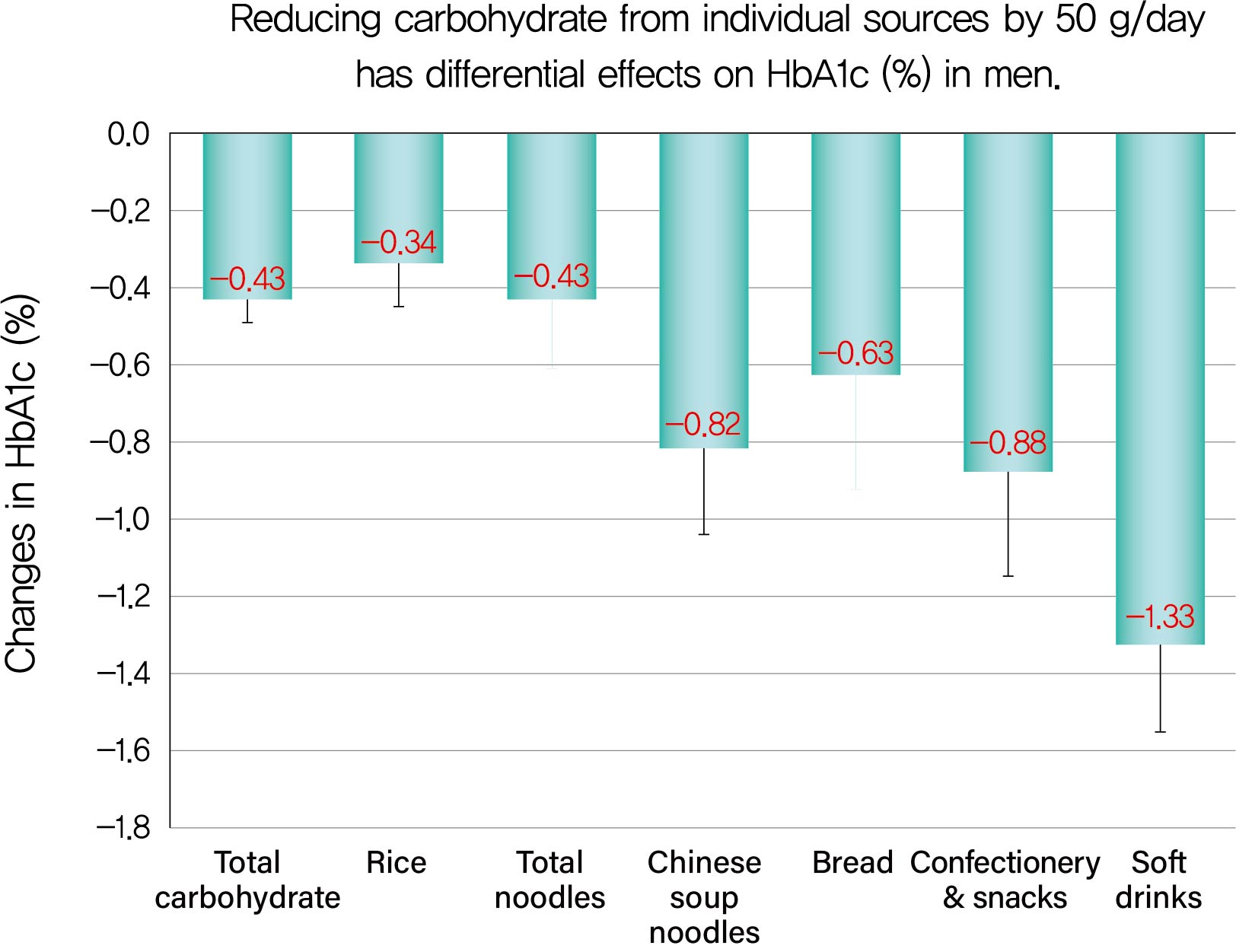
Background We evaluated decreases in glycosylated hemoglobin (HbA1c) achieved by reducing carbohydrate from various sources in type 2 diabetes mellitus patients.
Methods We followed up 138 male and 107 female outpatients on a moderate low-carbohydrate diet without diabetic medication for 6 months. Changes in carbohydrate sources (Δcarbohydrate) were assessed from 3-day dietary records at baseline and 6 months, and associations with changes in HbA1c (ΔHbA1c) were examined with Spearman's correlation coefficients (
r s) and multiple regression analysis.Results ΔHbA1c was −1.5%±1.6% in men and −0.9%±1.3% in women, while Δtotal carbohydrate was −115.3±103.7 g/day in men and −63.6±71.1 g/day in women. Positive associations with ΔHbA1c were found for Δtotal carbohydrate (
r s=0.584), Δcarbohydrate from soft drinks (0.368), confectionery (0.361), rice (0.325), bread (0.221), Chinese soup noodles (0.199) in men, and Δtotal carbohydrate (0.547) and Δcarbohydrate from rice (0.376) and confectionery (0.195) in women. Reducing carbohydrate sources by 50 g achieved decreases in HbA1c of 0.43% for total carbohydrate, 1.33% for soft drinks, 0.88% for confectionery, 0.63% for bread, 0.82% for Chinese soup noodles and 0.34% for rice in men and 0.45% for total carbohydrate, 0.67% for confectionery and 0.34% for rice in women, although mean reductions in carbohydrate from these sources were much smaller than that from rice.Conclusion Decreases in HbA1c achieved by reducing carbohydrate from soft drinks, confectionery, bread and Chinese soup noodles were 2- to 4-fold greater than that for rice. Our results will enable patients to decrease HbA1c efficiently (UMIN000009866).
-
Citations
Citations to this article as recorded by- Exploring diet associations with Covid-19 and other diseases: a Network Analysis–based approach
Rashmeet Toor, Inderveer Chana
Medical & Biological Engineering & Computing.2022; 60(4): 991. CrossRef - Comprehensive Understanding for Application in Korean Patients with Type 2 Diabetes Mellitus of the Consensus Statement on Carbohydrate-Restricted Diets by Korean Diabetes Association, Korean Society for the Study of Obesity, and Korean Society of Hyperte
Jong Han Choi, Jee-Hyun Kang, Suk Chon
Diabetes & Metabolism Journal.2022; 46(3): 377. CrossRef - Associations of Dietary Salt and Its Sources with Hemoglobin A1c in Patients with Type 2 Diabetes Not Taking Anti-Diabetic Medications: Analysis Based on 6-Month Intervention with a Moderate Low-Carbohydrate Diet
Hajime Haimoto, Takashi Murase, Shiho Watanabe, Keiko Maeda, Kenji Wakai
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2021; Volume 14: 4569. CrossRef
- Exploring diet associations with Covid-19 and other diseases: a Network Analysis–based approach
- Pathophysiology
- Relationships between Islet-Specific Autoantibody Titers and the Clinical Characteristics of Patients with Diabetes Mellitus
- Yiqian Zhang, Tong Yin, Xinlei Wang, Rongping Zhang, Jie Yuan, Yi Sun, Jing Zong, Shiwei Cui, Yunjuan Gu
- Diabetes Metab J. 2021;45(3):404-416. Published online July 21, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0239
- 6,369 View
- 161 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
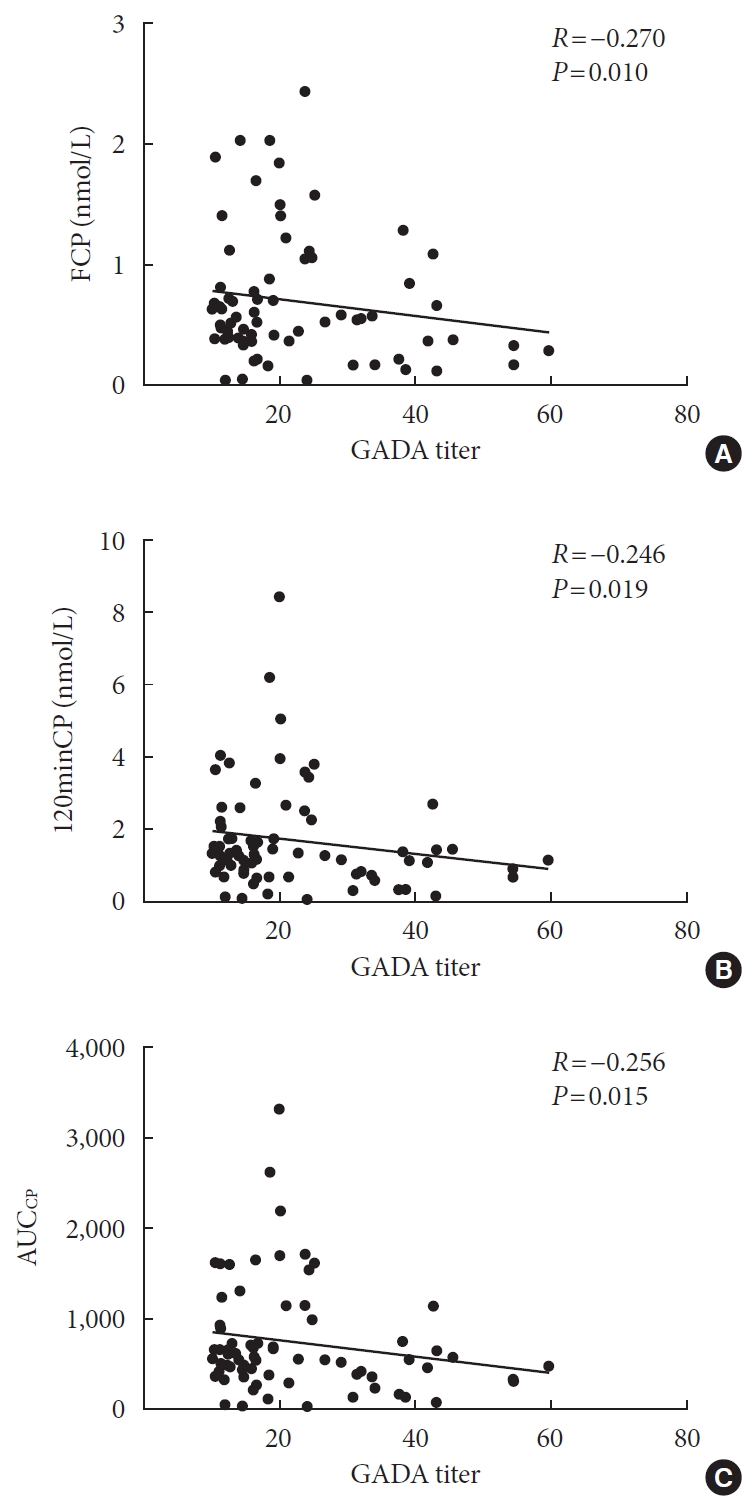
ePub Background Dysimmunity plays a key role in diabetes, especially type 1 diabetes mellitus. Islet-specific autoantibodies (ISAs) have been used as diagnostic markers for different phenotypic classifications of diabetes. This study was aimed to explore the relationships between ISA titers and the clinical characteristics of diabetic patients.
Methods A total of 509 diabetic patients admitted to Department of Endocrinology and Metabolism at the Affiliated Hospital of Nantong University were recruited. Anthropometric parameters, serum biochemical index, glycosylated hemoglobin, urinary microalbumin/creatinine ratio, ISAs, fat mass, and islet β-cell function were measured. Multiple linear regression analysis was performed to identify relationships between ISA titers and clinical characteristics.
Results Compared with autoantibody negative group, blood pressure, weight, total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), visceral fat mass, fasting C-peptide (FCP), 120 minutes C-peptide (120minCP) and area under C-peptide curve (AUCCP) of patients in either autoantibody positive or glutamate decarboxylase antibody (GADA) positive group were lower. Body mass index (BMI), waist circumference, triglycerides (TGs), body fat mass of patients in either autoantibody positive group were lower than autoantibody negative group. GADA titer negatively correlated with TC, LDL-C, FCP, 120minCP, and AUCCP. The islet cell antibody and insulin autoantibody titers both negatively correlated with body weight, BMI, TC, TG, and LDL-C. After adjusting confounders, multiple linear regression analysis showed that LDL-C and FCP negatively correlated with GADA titer.
Conclusion Diabetic patients with a high ISA titer, especially GADA titer, have worse islet β-cell function, but less abdominal obesity and fewer features of the metabolic syndrome.
-
Citations
Citations to this article as recorded by- Relationship between β-Cell Autoantibodies and Their Combination with Anthropometric and Metabolic Components and Microvascular Complications in Latent Autoimmune Diabetes in Adults
Tomislav Bulum, Marijana Vučić Lovrenčić, Jadranka Knežević Ćuća, Martina Tomić, Sandra Vučković-Rebrina, Lea Duvnjak
Biomedicines.2023; 11(9): 2561. CrossRef - RETRACTED ARTICLE: MiRNA-27a mediates insulin resistance in 3T3-L1 cells through the PPARγ
Yangming Zhuang, Ming Li
Molecular and Cellular Biochemistry.2022; 477(4): 1107. CrossRef - Correlation between Insulin Resistance and Microalbuminuria Creatinine Ratio in Postmenopausal Women
Han Na, Rong Wang, Hai-Long Zheng, Xiao-Pan Chen, Lin-Yang Zheng, Faustino R. Perez-Lopez
International Journal of Endocrinology.2022; 2022: 1. CrossRef - The longitudinal loss of islet autoantibody responses from diagnosis of type 1 diabetes occurs progressively over follow-up and is determined by low autoantibody titres, early-onset, and genetic variants
C L Williams, R Fareed, G L M Mortimer, R J Aitken, I V Wilson, G George, K M Gillespie, A J K Williams, Chitrabhanu Ballav, Atanu Dutta, Michelle Russell-Taylor, Rachel Besser, James Bursell, Shanthi Chandran, Sejal Patel, Anne Smith, Manohara Kenchaiah,
Clinical and Experimental Immunology.2022; 210(2): 151. CrossRef
- Relationship between β-Cell Autoantibodies and Their Combination with Anthropometric and Metabolic Components and Microvascular Complications in Latent Autoimmune Diabetes in Adults
- Pathophysiology
- Distinct Dose-Dependent Association of Free Fatty Acids with Diabetes Development in Nonalcoholic Fatty Liver Disease Patients
- Fuxi Li, Junzhao Ye, Yanhong Sun, Yansong Lin, Tingfeng Wu, Congxiang Shao, Qianqian Ma, Xianhua Liao, Shiting Feng, Bihui Zhong
- Diabetes Metab J. 2021;45(3):417-429. Published online March 15, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0039
- 5,692 View
- 154 Download
- 8 Web of Science
- 7 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
- Background
Excessive delivery of free fatty acids (FFAs) to the liver promotes steatosis and insulin resistance (IR), with IR defined as reduced glucose uptake, glycogen synthesis and anti-lipolysis stimulated by normal insulin levels. Whether the associations between FFAs and diabetes development differ between patients with and without nonalcoholic fatty liver disease (NAFLD) remains unclear.
Methods
Consecutive subjects (2,220 NAFLD subjects and 1,790 non-NAFLD subjects according to ultrasound imaging) were enrolled from the First Affiliated Hospital of Sun Yat-sen University between 2009 and 2019. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated.
Results
There was an approximate J-shaped relationship between FFA levels and HOMA-IR in the NAFLD group. Higher FFA concentration quartiles were associated with higher risks of IR (odds ratio [OR], 9.24; 95% confidence interval [CI], 6.43 to 13.36), prediabetes (OR, 10.48; 95% CI, 5.66 to 19.39), and type 2 diabetes mellitus (T2DM; OR, 19.43; 95% CI, 12.75 to 29.81) in the NAFLD group but not in the non-NAFLD group. The cut-off points for the FFA levels increased in a stepwise manner in discriminating IR, prediabetes and T2DM (573, 697, and 715 μmol/L) in the NAFLD group but not in non-NAFLD individuals.
Conclusion
A distinct dose-dependent relationship of FFA levels was found with IR, prediabetes and T2DM in NAFLD patients. Screening serum FFA levels in NAFLD patients would be valuable in preventing diabetes development. -
Citations
Citations to this article as recorded by- Mortality in metabolic dysfunction-associated steatotic liver disease: A nationwide population-based cohort study
Eugene Han, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Sang Hoon Ahn, Yong-ho Lee, Seung Up Kim
Metabolism.2024; 152: 155789. CrossRef - Global epidemiology of type 2 diabetes in patients with NAFLD or MAFLD: a systematic review and meta-analysis
Limin Cao, Yu An, Huiyuan Liu, Jinguo Jiang, Wenqi Liu, Yuhan Zhou, Mengyuan Shi, Wei Dai, Yanling Lv, Yuhong Zhao, Yanhui Lu, Liangkai Chen, Yang Xia
BMC Medicine.2024;[Epub] CrossRef - Metabolic Dysfunction-Associated Fatty Liver Disease and Mortality: A Population-Based Cohort Study
Kyung-Soo Kim, Sangmo Hong, Hong-Yup Ahn, Cheol-Young Park
Diabetes & Metabolism Journal.2023; 47(2): 220. CrossRef - Experimental model and novel therapeutic targets for non-alcoholic fatty liver disease development
Yujin Jin, Kyung-Sun Heo
The Korean Journal of Physiology & Pharmacology.2023; 27(4): 299. CrossRef - Triglyceride and glucose index is a simple and easy‐to‐calculate marker associated with nonalcoholic fatty liver disease
Kyung‐Soo Kim, Sangmo Hong, Hong‐Yup Ahn, Cheol‐Young Park
Obesity.2022; 30(6): 1279. CrossRef - Mongolian medicine in treating type 2 diabetes mellitus combined with nonalcoholic fatty liver disease via FXR/LXR-mediated P2X7R/NLRP3/NF-κB pathway activation
Shuyin Bao, Xiuzhi Wang, Qianqian Ma, Chengxi Wei, Jixing Nan, Wuliji Ao
Chinese Herbal Medicines.2022; 14(3): 367. CrossRef - Triglyceride Glucose-Waist Circumference Is Superior to the Homeostasis Model Assessment of Insulin Resistance in Identifying Nonalcoholic Fatty Liver Disease in Healthy Subjects
Hwi Seung Kim, Yun Kyung Cho, Eun Hee Kim, Min Jung Lee, Chang Hee Jung, Joong-Yeol Park, Hong-Kyu Kim, Woo Je Lee
Journal of Clinical Medicine.2021; 11(1): 41. CrossRef
- Mortality in metabolic dysfunction-associated steatotic liver disease: A nationwide population-based cohort study
- COVID-19
- Use of Renin-Angiotensin-Aldosterone System Inhibitors and Severe COVID-19 Outcomes in Patients with Hypertension: A Nationwide Cohort Study
- Jae Hyun Bae, Sun Kyu Choi, Nam Hoon Kim, Juneyoung Lee, Sin Gon Kim
- Diabetes Metab J. 2021;45(3):430-438. Published online February 22, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0279

- 7,922 View
- 296 Download
- 3 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
- Background
Angiotensin-converting enzyme 2 facilitates the entry of severe acute respiratory syndrome coronavirus 2 into the human body. We investigated the association of renin-angiotensin-aldosterone system (RAAS) inhibitor use with severe coronavirus disease 2019 (COVID-19) outcomes in hypertensive patients.
Methods
We identified hypertensive patients with confirmed COVID-19 from the Korean Health Insurance Review and Assessment Service from inception to May 15, 2020. The primary outcome was the composite of intensive care unit (ICU) admission, invasive mechanical ventilation (IMV), continuous renal replacement therapy (CRRT), extracorporeal membrane oxygenation (ECMO), and death from COVID-19. The individual components were evaluated as secondary outcomes.
Results
Of 1,374 hypertensive patients with COVID-19, 1,076 (78.3%) and 298 (21.7%) were users and never-users of RAAS inhibitors, respectively. The RAAS inhibitor users were not associated with the risk of the primary outcome (adjusted odds ratio [aOR], 0.72; 95% confidence interval [CI], 0.46 to 1.10). The risk of ICU admission was significantly lower in the users than the never-users (aOR, 0.44; 95% CI, 0.24 to 0.84). The RAAS inhibitors were beneficial only in ICU admissions that did not require IMV (aOR, 0.28; 95% CI, 0.14 to 0.58). The risk of death from COVID-19 was comparable between the groups (aOR, 1.09; 95% CI, 0.64 to 1.85). We could not evaluate the risks of CRRT and ECMO owing to the small number of events.
Conclusion
RAAS inhibitor use was not associated with the composite of severe outcomes in the hypertensive patients with COVID-19 but significantly lowered the risk of ICU admission, particularly in patients who did not require IMV. -
Citations
Citations to this article as recorded by- Systematic review and meta-analysis of the clinical outcomes of ACEI/ARB in East-Asian patients with COVID-19
Nancy Xurui Huang, Qi Yuan, Fang Fang, Bryan P. Yan, John E. Sanderson, Masaki Mogi
PLOS ONE.2023; 18(1): e0280280. CrossRef - Renin‐Angiotensin Aldosterone System Inhibitors and COVID‐19: A Systematic Review and Meta‐Analysis Revealing Critical Bias Across a Body of Observational Research
Jordan Loader, Frances C. Taylor, Erik Lampa, Johan Sundström
Journal of the American Heart Association.2022;[Epub] CrossRef - Renin-angiotensin-aldosterone system blockers in Bulgarian COVID-19 patients with or without chronic kidney disease
Rumen Filev, Lionel Rostaing, Mila Lyubomirova, Boris Bogov, Krassimir Kalinov, Dobrin Svinarov
Medicine.2022; 101(48): e31988. CrossRef
- Systematic review and meta-analysis of the clinical outcomes of ACEI/ARB in East-Asian patients with COVID-19
- Basic Research
- MondoA Is Required for Normal Myogenesis and Regulation of the Skeletal Muscle Glycogen Content in Mice
- Hui Ran, Yao Lu, Qi Zhang, Qiuyue Hu, Junmei Zhao, Kai Wang, Xuemei Tong, Qing Su
- Diabetes Metab J. 2021;45(3):439-451. Published online May 18, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0212
- Correction in: Diabetes Metab J 2021;45(5):797
- 6,162 View
- 191 Download
- 4 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub Background Skeletal muscle is the largest tissue in the human body, and it plays a major role in exerting force and maintaining metabolism homeostasis. The role of muscle transcription factors in the regulation of metabolism is not fully understood. MondoA is a glucose-sensing transcription factor that is highly expressed in skeletal muscle. Previous studies suggest that MondoA can influence systemic metabolism homeostasis. However, the function of MondoA in the skeletal muscle remains unclear.
Methods We generated muscle-specific MondoA knockout (MAKO) mice and analyzed the skeletal muscle morphology and glycogen content. Along with skeletal muscle from MAKO mice, C2C12 myocytes transfected with small interfering RNA against MondoA were also used to investigate the role and potential mechanism of MondoA in the development and glycogen metabolism of skeletal muscle.
Results MAKO caused muscle fiber atrophy, reduced the proportion of type II fibers compared to type I fibers, and increased the muscle glycogen level. MondoA knockdown inhibited myoblast proliferation, migration, and differentiation by inhibiting the phosphatase and tensin homolog (PTEN)/phosphoinositide 3-kinase (PI3K)/Akt pathway. Further mechanistic experiments revealed that the increased muscle glycogen in MAKO mice was caused by thioredoxin-interacting protein (TXNIP) downregulation, which led to upregulation of glucose transporter 4 (GLUT4), potentially increasing glucose uptake.
Conclusion MondoA appears to mediate mouse myofiber development, and MondoA decreases the muscle glycogen level. The findings indicate the potential function of MondoA in skeletal muscle, linking the glucose-related transcription factor to myogenesis and skeletal myofiber glycogen metabolism.
-
Citations
Citations to this article as recorded by- The Function of MondoA and ChREBP Nutrient—Sensing Factors in Metabolic Disease
Byungyong Ahn
International Journal of Molecular Sciences.2023; 24(10): 8811. CrossRef - Normal and Neoplastic Growth Suppression by the Extended Myc Network
Edward V. Prochownik, Huabo Wang
Cells.2022; 11(4): 747. CrossRef - The Role of Mondo Family Transcription Factors in Nutrient-Sensing and Obesity
Huiyi Ke, Yu Luan, Siming Wu, Yemin Zhu, Xuemei Tong
Frontiers in Endocrinology.2021;[Epub] CrossRef
- The Function of MondoA and ChREBP Nutrient—Sensing Factors in Metabolic Disease
- Lost in Translation? Measuring Diabetic Neuropathy in Humans and Animals (Diabetes Metab J 2021;45:27-42)
- Otto Jesus Hernandez Fustes
- Diabetes Metab J. 2021;45(3):452-453. Published online May 25, 2021
- DOI: https://doi.org/10.4093/dmj.2021.0020
- 2,830 View
- 64 Download

 KDA
KDA


 First
First Prev
Prev





